Stick an aquatic plant in an aquarium full of water and turn on
the lights. The plant is supplied with water, which provides ample
hydrogen and oxygen, and it has an energy source in the form of
the light falling on its leaves. Carbon is provided by carbon
dioxide diffusing into the water from the room air. These are the
three primary nutrients for aquarium plants.
Photosynthesis, the building of plant tissue using light energy, is a wonderful and complex process. The discussion that follows invokes some pretty hard-core biochemistry. More than any other section of this ebook, it is presented here for its intellectual interest rather than its practical applications. Don't be ashamed to skip ahead to where I discuss the proper lighting for an aquarium. You can always come back later to read this section. No one will ever know.
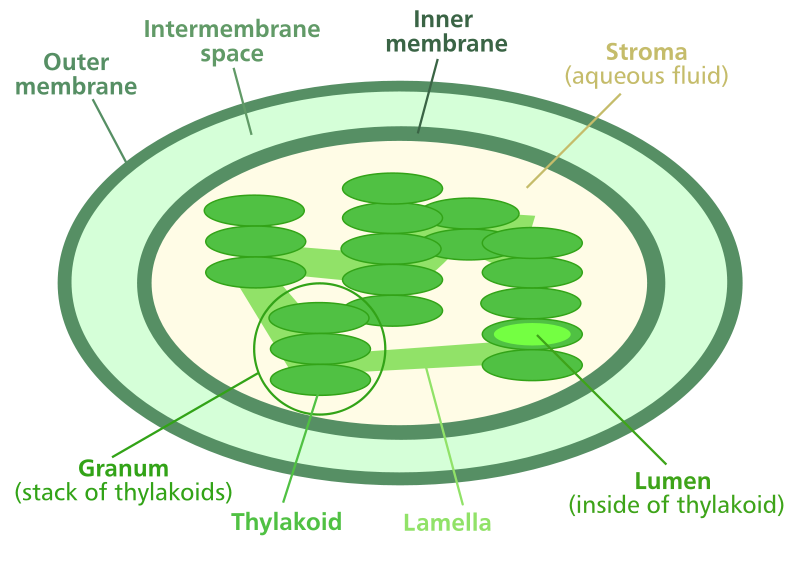
Photosynthesis takes place in aquarium plants in subcellular organelles called chloroplasts. These are found inside individual leaf cells and are about the size and shape of a bacterium. In fact, biologists now believe that chloroplasts were once a form of cyanobacterium, blue-green algae, that somehow became incorporated within the cells of the earliest ancestors of plants. Each chloroplast contains stacks of flat structures called thylakoids, which carry the chlorophyll and other pigments that are responsible for photosynthesis. Each thylakoid has an outer membrane enclosing an interior space called the lumen. The space within the chloroplast membrane, but outside the thylakoids, is called the stroma. The fluid filling the space within the cell but outside the chloroplasts is called the cytosol. All play a role in photosynthesis.
The photomicrograph at the top of the page shows a Vallisneria
leaf under magnification of 800x. The individual leaf cells are
visible, and each contains a large central vacuole
surrounded by numerous chloroplasts. The vacuole is a storage
organelle typically containing sugars.
The biological membranes separating the different compartments
are described as lipid bilayers. A lipid is a compound
composed mostly of carbon and hydrogen that is highly insoluble in
water. The lipids in a biological membrane are organized as pairs
of long fatty acid chains attached to a phosphate head that is
highly soluble in water. These phospholipids are easily
organized into a double layer, with the insoluble lipid tails
inside and the soluble phosphate heads facing outwards where they
are exposed to the surrounding watery fluid.
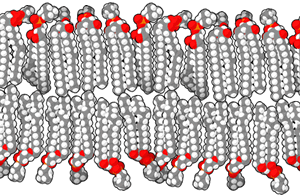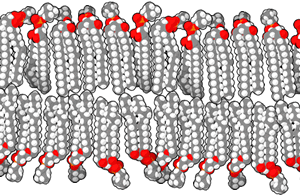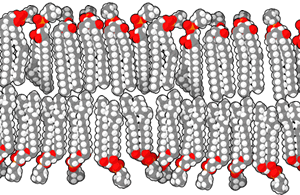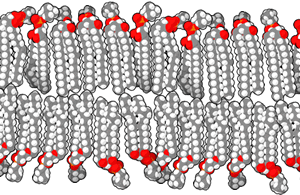
These lipid bilayers are very stable. They are further
strengthened by molecules like cholesterol and proteins that are
embedded in the layer. Cholesterol is itself a highly insoluble
lipid. Membrane proteins typically have a portion that is highly
insoluble and remains within the membrane, and one or two soluble
portions that protrude from one or both sides of the membrane into
the surrounding watery fluid. In effect, the lipid bilayer has wet
surfaces and a very dry interior, with water-soluble substances
preferring to be on the surfaces and insoluble substances
preferring to remain in the interior. Very few substances can
cross the entire membrane unassisted, so it is selectively
permeable, with protein "gateways" that allow only selected
molecules to cross.
The essence of photosynthesis is that light energy is used to
move protons from the stroma outside the thylakoids across the
thylakoid membrane to the lumen. This creates an electrical
voltage across the thylakoid membrane that powers a biological
motor embedded in the thylakoid membrane. This biological motor
stamps out molecules of ATP, the energy currency of living cells.
At the same time, electrons are stripped from water molecules
inside the lumen (generating oxygen gas as a byproduct) and used
to produce the reducing agent, NADPH, in the stroma. The chemical
energy carried by ATP and NADPH is then used to convert water and
carbon dioxide to carbohydrates, as well as carry out many other
chemical reactions essential to life.
Light energy comes in discrete packets or quanta called photons.
These are sometimes described as particles of light; while this is
not quite correct, it is close enough for our purposes. A photon
behaves like a ripple of elecromagnetic energy with a
characteristic wavelength, which lies between about 400 and 700
nanometers. That's 400 to 700 millionths of a millimeter. For
comparison, an atom of hydrogen has a diameter of about a
millionth of a millimeter. The amount of energy in a single photon
depends on its wavelength, with more energetic photons having
shorter wavelength. The energy also determines the color of
visible light; photons with a wavelength of 700 nanometers are
red, while those with a wavelength of 400 nanometers are
violet.
Wavelength
to color relationship
The energy of a photon ranges from 3 electron volts for a violet
photon to 1.8 electron volts for a red photon. The electron volt,
abbreviated eV, is a very small unit of energy suitable for
describing individual molecular reactions; a kilowatt-hour is
about 2.2 x 1025 eV. It takes over 13 eV to strip an
electron from a hydrogen atom in a vacuum, and most chemical bonds
in organic molecules can be broken with an energy of 3 to 5 eV.
Ordinary thermal motion at aquarium temperature has an energy
averaging about 0.04 eV per molecule, far less than what is
required to break chemical bonds, which is why most molecules are
stable under normal conditions.
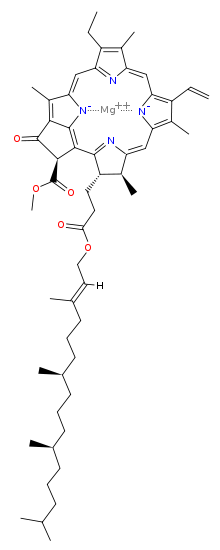
The chief light-gathering molecule in plants is chlorophyll. Chlorophyll comes in several varieties, but all are based on a ring of linked carbon atoms forming a kind of flat donut called porphyrin. The hole at the center of the porphyrin is lined by four nitrogen atoms bonded to the carbon backbone of the porphyrin and these four nitrogen atoms, in turn, hold in place a single magnesium atom that is the reactive heart of the molecule. Most varieties of chlorophyll also have a hydrocarbon chain forming a kind of "tail" to the molecule.

Clorophyll A
Chlorophyll gets its distinctive green color because it absorbs
red light most strongly. It also absorbs blue and violet
light. The green light it fails to absorb is reflected to
our eyes.
Additional light is absorbed by carotenoids, such as beta-carotene.

Carotenoids tend to absorb mostly blue light and so are yellow to
red in color. They may help prevent damage to chlorophyll caused
by intense light by "discharging" chlorophyll that picks up too
much energy. This process is called quenching.
Clorophyll and carotenoids are arranged into structures called photosystems
that sit astride the thylakoid membrane. There are two kinds of
photosystems in green plants, called Photosystem I and Photosystem
II. Each consists of a pair of protein molecules that form a kind
of scaffolding for positioning as many as 200 chlorophyll and
carotenoid molecules -- the antenna molecules of the
photosystem. These gather light energy and funnel it to the reactive
center of the photosystem. The reactive center is itself a
pair of chlorophyll molecules. While the reactive center is
capable of absorbing photons, most are absorbed somewhere in the
antenna array. The absorbed energy kicks an electron off the pair
of chlorophyll molecules at the reactive center.
Near the reactive center of Photosystem II (PII) are a pair of
molecules of a highly modified form of chlorophyll called
pheophytin.

Pheophytin is essentially chlorophyll in which the magnesium has
been replaced by a pair of hydrogen atoms. This molecule readily
picks up the energetic electron ejected from the reactive center
and acts like an electrical conduit, moving the electron away from
the reactive center to a molecule of plastoquinone bound
to the reactive complex.

Plastoquinone has the property that either of the oxygen atoms
double bonded to the carbon ring readily picks up an electron.
A PII complex has two molecules of plastoquinone attached to it. The first plastoquinone is firmly bonded in place, while the second is quite loosely attached. When the first plastoquinone receives the high-energy electron from the pheophytin, it immediately passes it on to the second plastoquinone. This plastoquinone is positioned near the outer surface of the thylakoid membrane, where it is sticks out into the stroma of the chloroplast. The extra electron on the oxygen atom of the second plastoquinone pulls a proton off a nearby hydronium ion, converting the plastoquinone to plastosemiquinone. When a second elecron reaches the plastosemiquinone, it is converted to plastoquinol.

At this point the plastoquinol comes loose from PII and is
replaced by a fresh plastoquinone. The plastoquinol is highly
insoluble in water and so remains embedded in the thylakoid
membrane, but is free to move within the membrane. We'll come back
to its fate presently.
When the reaction center gives up its high-energy electron, it is
left with a positive charge that is concentrated on one of the two
chlorophyll molecules. Near the reaction center, on the side of
the PSII that faces the lumen of the thylakoid, is an oxygen-evolving
complex. This is a cluster of four manganese atoms and one
calcium atom bonded together by oxygen atoms. Through a mechanism
that is not fully understood and has never been artificially
duplicated, this complex splits water molecules, producing oxygen,
hydronium, and electrons. The oxygen escapes from the thylakoid
and, ultimately, from the plant, joining our planet's great pool
of atmospheric oxygen. The electron is passed to the reaction
center, neutralizing its positive charge and preparing it to
generate another high-energy electron. The hydronium is released
into the lumen of the thylakoid.
The net result of everything that has taken place so far is that
two water molecules have been destroyed; an oxygen molecule has
been released and leaves the chloroplast; four protons have been
removed from the stroma and four protons have been added to the
lumen; and two plastoquinones have been reduced to plastoquinol in
the thylakoid membrane. What does this accomplish? The
plastonquinol is a carrier of high-energy electons, making it a reducing
agent useful for driving chemical reactions. These include
reducing carbon dioxide to carbohydrates, which we'll come to
later. Protons are building up inside the lumen and are being
depleted in the stroma, creating a proton gradient across
the thylakoid membrane. In other words, the light energy captured
by the PII has been transformed into chemical energy in the form
of plastoquinol and electrical energy in the form of the proton
gradient across the thylakoid membrane.
The electrical energy stored in the proton gradient is not usable
for most chemical reactions. Further steps are needed to
convert this energy to a more useful form. We'll discuss how that
is done later on.
The chemical energy in plastoquinol is also problematic.
Plastoquinol is extremely insoluble in water. The molecule is
mostly a backbone of carbon studded with hydrogen atoms and in
such compounds, the proton is buried much deeper in the electron
cloud surrounding the molecule than a proton on the surface of a
water molecule. Water molecules are not at all interested in
getting close to such molecules. In effect, plastoquinone and
plastoquinol are big, greasy molecules that cannot leave the dry
environment of the thylakoid membrane. What is needed is a
water-soluble carrier of high-energy electrons that can leave the
membrane and be used elsewhere in the chloroplast.
This is produced by an electron transport chain in the thylakoid membrane. Strictly speaking, this chain began with the pheophytin and continued with the plastoquinone and plastoquinol. Next in the chain is a very large molecular structure called cytochrome b6f. This is also known as plasmoquinol - plasmocyanin reductase. This sits astride the thylakoid membrane and consists of two protein clusters, each containing eight protein strands, several of which either contain one or two heme groups or an iron-sulfur cluster.

Heme
Heme is based on a porphyrin, like chlorophyll, but it has an iron atom at its center rather than a magnesium atom. Iron is a particularly good atom for temporarily storing an electron, and the heme likely serves this function in cytochromes. Some of the proteins in cytochrome b6f trap a string of water molecules that can act as a "proton wire", because of the ability of a proton to jump from water molecule to water molecule with relative ease. All these features help the cytochrome b6f to carry out its functions.
The cytochrome b6f
carries out a kind of shuffle with plasmoquinol and plasmoquinone.
It first strips two electrons from a plasmoquinol molecule on the
lumen side of the thylakoid membrane, converting the plasmoquinol
to plasmoquinone and ejecting two protons into the lumen. The
plasmoquinone is then free to go find a PII to dock with and pick
up two more electrons. Meanwhile, the two energetic electrons
pulled off the plastoquinol follow different paths.
The first electron was pulled off with moderate energy, and it is
used to convert a plasmoquinone near the stroma side of the
thylakoid membrane to plasmosemiquinone. In the process, a proton
is picked up from the stroma.
The second electron has higher energy and is directed towards a
molecule of plastocyanin on the lumen side of the
thylakoid membrane. This consists of a strand of protein wrapped
around a copper atom. Copper, like iron, can pick up and store an
electron. The reduced plastocyanin then comes undocked from the
cytochrome b6f and is
replaced with an oxidized plastocyanin.
Another plastoquinol docks with the cytochrome b6f , and the process completes, except that the low energy electron finishes oxidizing the plastosemiquinone from the first cycle to plastoquinone. Again, a proton is picked up from the stroma, and two protons are released into the lumen.
The net result of all this is that the cytochrome b6f converts a molecule of plastoquinol back to plastoquinone, reduces two molecules of plastocyanin on the lumen side of the membrane, and pumps four protons from the stroma to the lumen. Reducing power has been transferred from plastoquinol to plastocyanin and the proton gradient across the thylakoid membrane has been further increased.
Plastocyanin is water-soluble, a big advantage, but it is a big
honking molecule too unwieldy for most reactions. It's also on the
wrong side of the thylakoid membrane. Nature's solution to this
problem is to reenergize the electron carried by plastocyanin,
using a second photosystem, PI, and transfer the electron to a
much smaller water-soluble molecule, NADPH, that is on the stroma
side of the membrane and makes a splendid general-purpose reducing
agent.
PI is similar in structure to PII; it has an antenna array and a reaction center. However, in place of the oxygen evolving complex of PII, PI has a binding site for plastocyanin that extracts its electron to recharge the active site, and it passes the reenergized electrons through chlorophyll and phylloquinone (vitamin K1) to an iron-sulfur complex. The iron-sulfur complex in turn reduces ferredoxin, a water-soluble enzyme on the stroma side of the thylakoid membrane, which contains its own iron-sulfur cluster to store the electron. The ferredoxin transports the electron to an iron-sulfur enzyme in the stroma, ferredoxin-NADP+ reductase, which transfers the electron to nicotinamide adenine dinucleotide phosphate, NADP+.
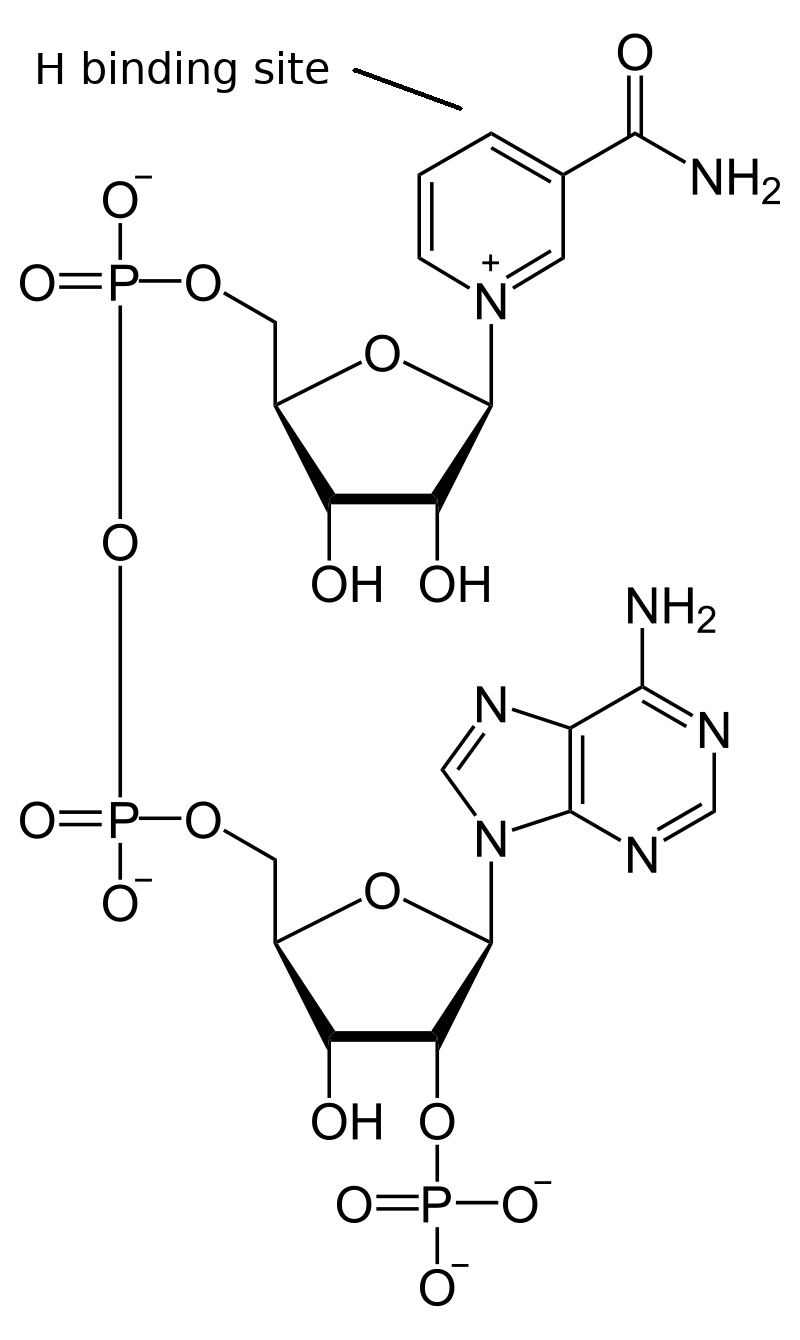
When NADP+ is given an electron, it picks up a proton from a nearby hydronium to form NADPH. The proton and electron bind as a hydrogen atom to the uppermost ring of the NADP+ molecule shown in the preceding diagram. NADPH is one of the general-purpose reducing agents used in chemical reactions throughout living cells. It is a small, water-soluble molecule ideal for this purpose.
This still leaves the question of how to exploit the energy stored in the proton gradient. This is done using a marvelous molecular machine, ATP synthase. This structure is a molecular motor, complete with a rotor that takes in phosphate and APD and stamps them together to form ATP. The motor is driven by a current of protons passing from the proton-rich lumen to the proton-poor stroma. ATP, as a small, water-soluble molecule, is an ideal energy currency for the cell.
ATP synthase is also found in mitochondria, which are organelles
remarkably similar to chloroplasts. However, mitochondria build up
their proton gradient for ATP synthesis by oxidizing pyruvate from
carbohydrates or other food molecules. Mitochondria are present in
the cells of all air-breathing organisms, including plants, which
use mitochondria to produce ATP from their starch reserves during
hours of darkness or in parts of the plant that do not
photosynthesize (such as the roots.)
Somewhat technical
video on ATP synthase
The overall reaction is:
2 H2O + 2 NADP+ + 3 ADP + 3 Pi + light → 2 NADPH + 2 H+ + 3 ATP + O2
This is the kind of photosynthesis that takes place in plants and
the closely related green algae.
Photosynthesis takes place in a slightly different manner in cyanobacteria (blue-green algae), which have two additional pigments, phycoerythrin and phycocyanin. Phycocyanin is good at absorbing orange and red light, while phycoerythrin is good at absorbing bluish-green through yellow light. These pigments are water-soluble, unlike chlorophylls and carotenoids,and so they sit on the outer membrane of the thylakoid in a structure called a phycobilisome. Light absorbed by phycobilisomes is transferred to the chlorophyll in PII. When cyanobacteria were captured by larger cells and became chloroplasts, those chloroplast-bearing cells that evolved to become green algae and green plants lost the ability to produce phycobilisomes. Those that evolved to become red algae retained the ability to use phycoerythrin and phycocyanin, which gives red algae their color. Brown algae and diatoms lost their ability to produce phycobilisomes but evolved the ability to use a carotene-like compound called phycoxanthin in their antenna arrays that is good at absorbing blue and green light.
The ability of some algae to use light wavelengths that green plants cannot use efficiently means that quality of lighting in a planted aquarium can affect which kinds of algae appear. Lighting rich in the wavelengths that cannot be absorbed by green plants favors the growth of cyanobacteria, red and brown algae, and diatoms. On the other hand, green algae like the same kind of lighting as green plants, which is why green hair algae and other green algae are difficult to completely eradicate from a tank in which green plants are flourishing. I'll have more to say about lighting later on.
As a matter of curiosity, there are other families of bacteria
besides cyanobacteria that carry out photosynthesis, but only
under conditions of very low oxygen. These include the green and
purple sulfur bacteria, which use hydrogen sulfide in place of
water as an electron source. Because it requires much less energy
to split hydrogen sulfide than water, these bacteria have only one
form of photosynthetic complex -- PII in the case of purple sulfur
bacteria and PI in the case of green sulfur bacteria. The extra
"boost" given by the second photosynthetic complex is not needed
to split hydrogen sulfide. There are also purple and green
nonsulfur bacteria that use hydrogen as an electron source.
The photosynthesis process we've just looked at produces three
ATP for every two NADPH. It turns out this is richer in NADPH than
most cells require. As a result, NADP+ becomes scarce
in an actively photosynthesizing cell. This causes reduced
ferredoxin to accumulate at the expense of oxidized ferredoxin.
When the supply of oxidized ferredoxin becomes low, PI can no
longer transfer its energetic electron to oxidized ferredoxin.
Instead, it begins transferring it to plastoquinone instead,
producing plastoquinol that feeds back into cytochrome b6f.
This means that the electrons perpetually cycle between cytochrome
b6f and PI, building up the
proton gradient but generating no NADPH. This allows ATP to be
synthesized without NADPH, bringing the mixture of photosynthetic
products down to what the cell needs.
Because the PII is cut out of this cycle, this form of
photosynthesis generates no oxygen.
Some purple and green nonsulfur bacteria use only the cyclic
pathway. We'll see shortly that NADPH is required to fix carbon
dioxide, so these bacteria cannot get their carbon from the air.
They must use organic carbon compounds as a carbon source.
Now that the cell has chemical energy and reducing power, in the
form of ATP and NADPH, the next step is to take carbon dioxide
from the aquarium water and reduce the carbon to a biologically
useful form. This set of reactions are known as the dark
reactions of photosynthesis, since they take place without
further input of light energy.
Carbon dioxide is processed by green plants through a cycle of chemical reactions called the Calvin cycle.* The first step of this cycle involves an enzyme present in the stroma of chloroplasts called ribulose-1,5-bisphosphate carboxylase, or RuBisCo for short. This is a clump of sixteen protein chains wrapped around a magnesium ion. The magnesium ion acts as a kind of chemical lever, forcing a carbon dioxide molecule close enough to a nearby nitrogen atom in the protein that the two bond. The protein then transfers the carbon dioxide to a molecule of ribulose 1,5-bisphosphate. This is a simple sugar, based on a chain of five carbon atoms, that the chloroplast has rendered more chemically reactive by attaching a phosphate to each end of the molecule.


While the mechanism whereby a molecule is activated by adding a
phosphate group is not always known, it seems reasonably clear in
this case. Remember that phosphate is typically singly or doubly
ionized at neutral pH. The pumping of protons from the stroma to
the lumen makes the stroma alkaline, further tending to ionize
phosphate, so that it will typically be doubly ionized in this
environment. The two negative charges at each end of the ribulose
1,5-bisphosphate strongly repel each other, so that the molecule
is effectively being pulled from both ends. This makes it that
much easier to break the molecule by inserting a carbon dioxide
molecule.
Some of the 3-phosphoglycerate is phosphorylated by the enzyme,
phosphoglycerate kinase, which obtains the phosphate by converting
an ATP to ADP. Magnesium is required to permit the phosphorylation
to proceed, probably by shielding the charge of the phosphate
group. This seems to be a common pattern in phosphorylating
enzymes. The product is the molecule,
1,3-bisphosphoglyceric acid, which is energetically primed for
further reactions.

Next, the enzyme, glyceraldehyde 3-phosphate dehydrogenase, uses
an NADPH to reduce this molecule to glyceraldehyde 3-phosphate,
releasing a phosphate in the process.

The effect is that an oxygen atom has been stripped off the
3-phosphoglycerate, a classic reduction reaction, using the energy
of ATP and the reducing power of NADPH. This is the point where
the carbon from atmospheric carbon dioxide is actually reduced.
However, if the process ended here, then the supply of ribulose
1,5-bisphosphate would soon be depleted and carbon fixation would
crash to a halt. Most of the glyceraldehyde 3-phosphate is used to
regenerate ribulose 1,5-bisphosphate, with only a fraction being
siphoned off to supply the cell's other carbohydrate needs.
The regeneration process begins as the enzyme, triose phosphate isomerase, converts some of the gluceraldehyde 3-phospate to dihydroxyacetone phosphate.
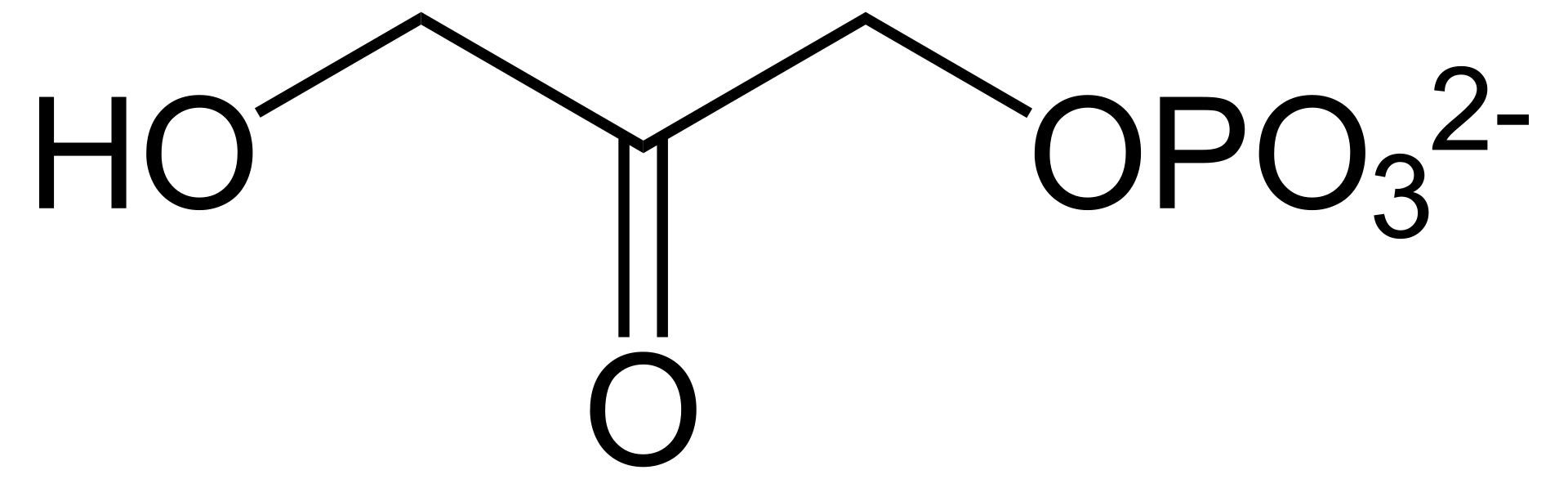
This means shifting a proton from from one oxygen to a neighboring oxygen, a process that requires almost no energy expenditure. All the enzyme does is give the reaction a nudge, making it go considerably faster than it would without the enzyme.
The enzyme, fructose-bisphosphate aldolase, then combines some of the dihydroxyacetone phosphate with some of the remaining gluceraldehyde 3-phosphate to form fructose 1,6-bisphosphate.

This is another reaction requiring very little energy that just
needs a nudge from the enzyme. In fact, the process is reversed in
the dark, so that the same enzyme splits fructose 1,6-bisphosphate
into gluceraldehyde 3-phosphae and dihydroxyacetone
phosphate to be oxidized for energy. Fructose 1,6-bisphosphate is
the one of the most common forms of simple carbohydrate in all
living cells. It's the form that glucose and fructose are
converted to almost at once when they are absorbed by animal
cells.
At this point in the cycle, six molecules of ribulose
1,5-bisphosphate and six molecules of carbon dioxide have been
converted to four molecules of fructose 1,6-bisphosphate, two
molecules of gluceraldehyde 3-phosphate, and two molecules of
dihydroxyacetone phosphate. One molecule of fructose
1,6-bisphosphate is siphoned off to be used elsewhere in the cell.
The other three molecules of fructose 1,6-bisphosphate and the
remaining gluceraldehyde 3-phosphate and dihydroxyacetone
phosphate are used to regenerate ribulose 1,6-bisphosphate. This
requires an elaborate chemical ballet to convert three six-carbon
sugars and four three-carbon sugars to six five-carbon sugars.
First the enzyme, fructose 1,6-bisphosphatase, splits off one of
the phosphates of a fructose 1,6-bisphosphate to produce fructose
6-phosphate.

Next the fructose 6-phosphate has two carbons and their associated oxygen and hydrogen transferred to gluceraldehyde 3-phosphate by an enzyme called transketolase. The products are erythrose 4-phosphate and xylulose 5-phosphate:
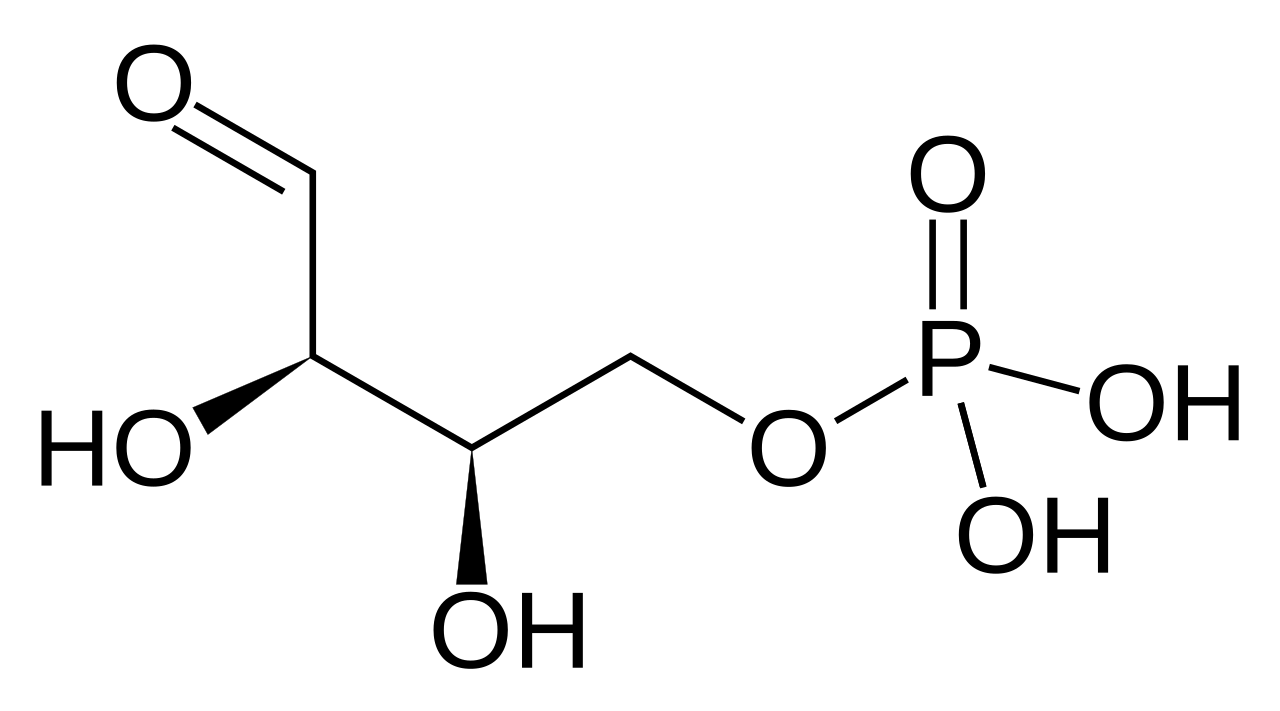
This is another reaction needing just a nudge from the enzyme.
The erythrose 4-phosphate is now combined with dihydroxyacetone
by aldolase to produce sedoheptulose-1,7-bisphosphate. This in
turn is stripped of a phosphate by
sedoheptulose-1,7-bisphosphatase to produce
sedoheptulose7-phosphate. The removal of the phosphate is a kind
of ratchet ensuring the reaction only goes in one direction.

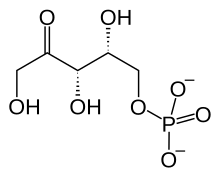
This is worked over by transketolase again, which strips two carbons and their associated oxygen and hydrogen and transfers them to yet another gluceraldehyde 3-phosphate to produce ribose-5-phosphate and another xylulose 5-phosphate:

This is nudged by phosphopentose isomerase to become ribulose 5-phosphate
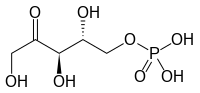 680
680
which then receives a phosphate group from an ATP via
phosphoribulokinase to become ribulose 1,5-bisphosphate, ready to
begin the cycle again. The final loose end is the xylulose
5-phosphate. This is converted to ribulose 5-phosphate by
phosphopentose epimerase, feeding back into the cycle a couple of
steps back.
So there's a lot going on here, as the chloroplast shuffles carbons around, adding energy as needed using ATP. The overall reaction is
6 CO2 + 12 NADPH + 12 H+ + 18 ATP → fructose 1,6-bisphosphate + 12 NADP+ + 18 ADP + 6 H2O + 16 PO43-
Carbon dioxide from the aquarium water, electrons supplied by
NADPH, and energy supplied by ATP go in, and fructose
1,6-bisphosphate and spent NADP+ and ADP come out.
The fructose 1,6-bisphosphate can be converted to fructose 6-phosphate by fructose 1,6-bisphosphatase, to glucose 6-phosphate by glucose 6-phosphate isomerase, then to glucose by glucose-6-phosphatase. Glucose is the building block for starch and cellulose, which are respectively the chief energy storage and structural molecules in aquarium plants.
A number of aquarium companies market products advertised as "liquid carbon" that purport to supply carbon to aquarium plants in dissolved form rather than by carbon dioxide injection. These all consist of various forms of glutaraldehyde.
Glutaraldehyde is used as a sterilant in hospitals, medical clinics, and dentist's offices. A sterilant is a disinfectant powerful enough to destroy all microbial life exposed to it, including viruses and bacterial endospores. The oxygen atoms exposed at the two ends of the molecule are highly reactive and allow glutaraldehyde to cross-link proteins in cells, which screws them up enough to kill the cell. The pure chemical is reactive enough that it is sometimes used to remove warts.
Is this something you want in your aquarium? Pharmacists have an
old saying, "the dose makes the poison", and the risks should not
be exaggerated. The glutaraldehyde in "liquid carbon" products is
highly diluted, with typical concentrations in the commercial
product of 1% to 4%. The glutaraldehyde may also be chemically
modified to make it less toxic than straight glutaraldehyde. For
example, one commercial product iis described as 2.5%
polycycloglutaracetal. It turns out that you will not find the
term "polycyclogutaracetal" anywhere but in literature describing
the commercial product or aquarium keeper forums trying to figure
out what this means. It is not a chemical name recognized by
organic chemists. Glutaraldehyde does have some tendency to
polymerize at high pH, and my guess is that the product is
basically glutaraldehyde in an akaline solution that has partially
polymerized.
At normal tank doses, the concentration will be about 3 ppm. This
is ten times the concentration of glutaraldehyde that is known to
be irritating to the skin. It also corresponds to a carbon dioxide
concentration of about 7 ppm. This is much higher than the carbon
dioxide concentration in equilibrium with room air, but lower than
typical injected carbon dioxide concentrations, and that's
assuming it's as available to plants as carbon dioxide is. There
is no reason to think so. Glutaraldehyde is not one of the
photosynthetic intermediate compounds in the Calvin cycle we just
described, so it is not obvious how it would insert itself into
the cycle.
The bottom line is that it is highly dubious that "liquid carbon"
actually supplies plants with significant carbon above that which
is present in equilibrium with room air. It certainly is not
comparable with carbon dioxide injection. Such benefits as it
seems to confer most likely come from its effectiveness as an
algicide, as I will discuss in the section on aquarium pests.
Both quantity and quality of light are important.
The following curve shows why quality of light is important.
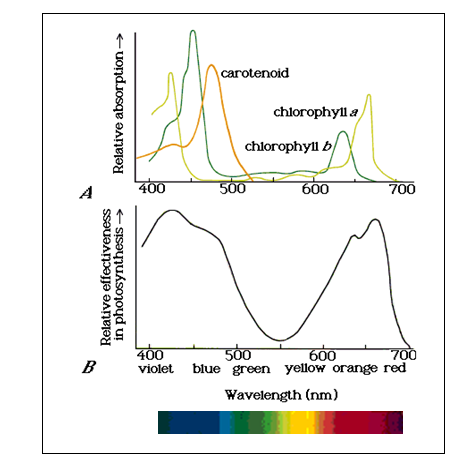
This shows the absorption of different pigments present in green plants and the resulting effectiveness of photosynthesis. You can see that photosynthesis is most effective in a range from deep violet to sky blue and from orange to red. Green through yellow light is relatively ineffective in driving photosynthesis. This has two important implications.
Ultimately, the lighting in an aquarium is drawing on some energy
source, usually electric current. Electrical energy that is
converted to yellow-green light is mostly wasted in
photosynthesis. However, this is precisely the region of the
spectrum where human eyesight is most sensitive. A light source
that produces only violet, blue, orange, and red light has a
fairly dreadful violet-pink color under which plants and fish are
not particularly attractive. In particular, the very colors that
make plants look beautiful are precisely the colors the plants are
not absorbing!
Since an aquarium is meant to be visually pleasing, we must make
compromises. There must be sufficient yellow-green light to make
the aquarium attractive without wasting too much energy (or
promoting algae growth, as I'll discuss in the next section.)
Fortunately, the fact that our eyes are most sensitive to
these non-photosynthetic colors means that we can bump up the
photosynthetic colors quite a lot before there is a noticeable
degradation in appearance.
The other implication of the inability of green plants to use yelllow-green light efficiently is illustrated by the next curve.
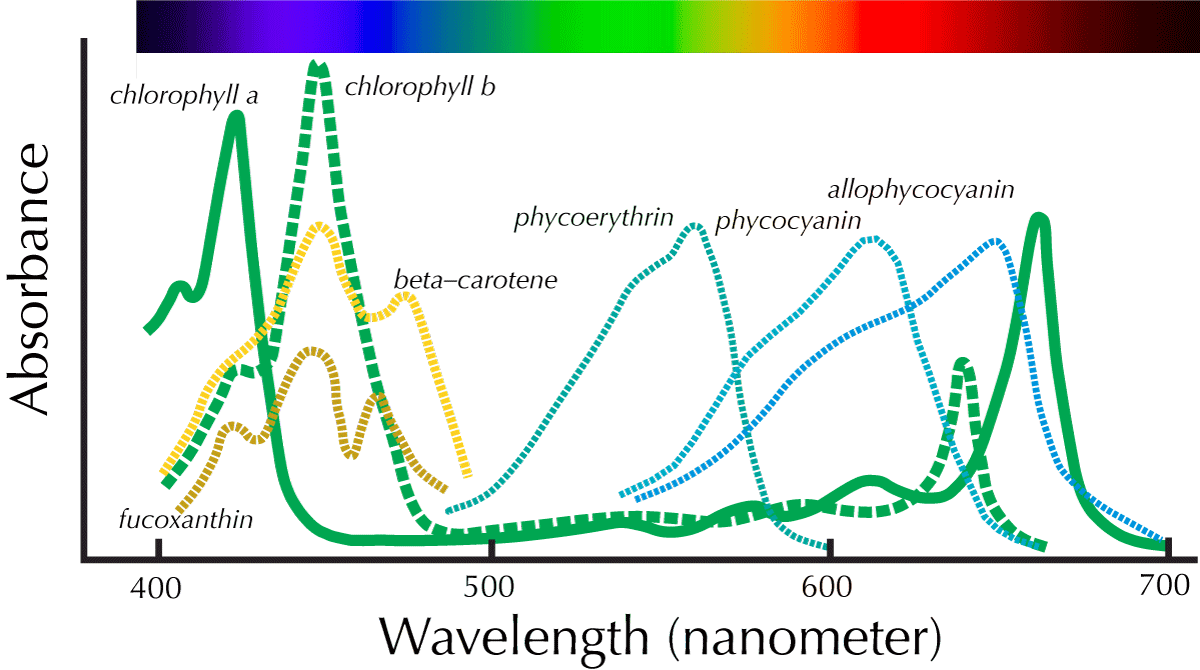
This graph contrasts the absorption of chlorophyll and carotenoids with the additional photosynthetic pigments found in algae. Fucoxanthin is characteristic of brown algae and diatoms, and it is somewhat more effective at absorbing blue-green light than chlorophyll. The effect is small enough that quality of light is not usually the main issue behind a diatom infestation.
Phycocyanin and phycoerythrin are characteristic of cyanobacteria and red algae. The latter includes black brush algae, one of the less welcome guests in an aquarium. Phycocyanin absorbs light ranging in color from yellow-green to orange, while phycoerythrin absorbs light from green to yellow. Phycoerythrin, in particular, absorbs light right in the range that the photosynthetic pigments of green plants cannot. This means that aquarium lighting rich in yellow-green light gives an advantage to red algae and cyanobacteria.
There are two aspects to quantity of light: intensity and duration. The intensity of light is how much light energy of different colors falls on a given area per unit of time. The duration, or photoperiod, is what fraction of a day the light is shining.
A complete scientific description of the intensity of light is given by the power per unit area per unit wavelength at each wavelength. This describes just how much energy is falling on an illuminated surface, such as a leaf, each second and at which colors. However, it takes a fairly sophisticated scientific instrument (a spectrophotometer) to obtain this data. Such instruments are far outside the budget of most home aquarium keepers, with basic portable spectophotometers carrying a price tag in excess of $3500 US. Nor would the information obtained be of immediate use for predicting the effects on plants, since it must then be adjusted for the photosynthetic effectiveness at each wavelength.
The practical alternative for a home aquarium keeper is a PAR
meter. These are much more affordable, with a price as low as $120
US for the most basic models. A PAR meter measures the light
intensity as a single number called the photosynthetic photon flux
density or PPFD. This is an actual count of photons falling on a
surface, expressed as micromoles per square meter per second. It's
an imperfect measure, since it includes all photons between 400
and 700 nanometers (deep violet to red) and weighs them equally.
Thus, it does not take into account the poor response to
yellow-green photons at 550 nm. The more precise quantity is known
as yield photon flux, YFD, which is weighted for photosynthetic
response and includes photons outside the 400 nm to 700 nm range.
PPFD is converted to YFD using a quality factor that is based on
the nature of the light source, but this is imprecise.
For practical purposes, for the home aquarium keepers, PAR is
almost certainly good enough. There is some indication from
controlled experiments that light quality is not that critical to
plant growth, so long as overall intensity is adequate. And it
turns out that there are so many other variables involved that
answering the question "How much light do I need in my aquarium?"
is more art than science, even if you have an accurate measurement
of YFD.
If even a PAR meter is outside your budget -- and it is reasonable to question whether this is a good investment for an individual aquarium keeper -- the alternative is to estimate PAR by calculating the illumination of your tank and making a suitable conversion. Illumination is measured in lumens per square meter, where lumen is a measure of power adjusted for human visual response. In other words, it's a measure intended for estimating how well a light source lights up a room for human eyes. We have already seen that this is not the same as how well a light source produces photosynthesis in plants. So any estimate of YFD based on illumination is going to involve poorly-defined quality factors, to the point that many hardcore aquarium keepers regard illumination calculations as all but useless.
I am not willing to go quite that far. In our day of high consciousness of energy efficiency, practically all light sources you can purchase will have a published lumen output. It is then fairly simple to estimate the area on which that light will shine to obtain the illuminance and then apply a quality factor to estimate PAR. In fact, there is a fairly decent calculator for doing so here. For all the uncertainty in precisely how this converts to PAR, it is at least a good starting estimate that will get you in the ballpark. And "in the ballpark" may be as much as you can hope for anyway.
I hesitate to recommend that individual aquarium keepers purchase
their own PAR meter for another reason: Once you have a good
lighting system in place, your PAR either does not change much (if
you are using LED lighting) or changes in a fairly predictable way
(if you are using fluorescent lighting, which degrades fairly
predictably over time.) This means that it makes much more sense
to borrow rather than purchase a PAR meter for the small number of
measurements you need to set up your tank. If you can find a local
aquarium club, it may very well own a PAR meter that can be loaned
out to members of the club.
LED lights last many years if they are protected from excessive moisture and are adequately cooled. The best LED fixtures feature a mixture of colors that cover the photosynthetic spectrum while providing enough yellow-green light for plants and fish to look their best. Fluorescent bulbs must be changed regularly, since they begin to lose intensity significantly after about six months. Many aquarium keepers combine an actinic ("daylight") bulb with a plant bulb optimized for the photosynthetic spectrum ("grow bulb") in a double bulb fixture. Both actinic and grow bulbs are readily available at fish shops or on the Internet, but with the declining prices and growing popularity of LEDs, it is likely that LED fixtures will dominate the hobby in the future.
Not all fluorescent bulbs are created equal. Look for T5 HO
bulbs. The HO stands for "high output" and these produce a lot of
blue light that really gets photosynthesis going. Manufacturers
claim that the bulbs lose less than 10% of their output intensity
over their lifetime, which is claimed to be 20,000 hours. Assuming
the claims are good, that means you don't need to replace bulbs
periodically due to intensity fade, and a bulb will last over six
years before burning out. Sounds too good to be true? Yeah, I
wonder, too. But I've had good results with them so far.
Duration is simple enough: How many hours per day is the tank
illuminated? Most aquarium plants are from the tropics or
subtropics, and so experience a period of full daylight of around
ten hours per day. This is in contrast with temperate zone plants,
whose active growing season coincides with daylight hours of up to
14 hours per day. Temperate zone plants are adapted to take full
advantage of long days, but tropical plants are not.
Just what the implications are for plant growth remain uncertain. Some temperate to subtropical plants will bloom only when the day length exceeds a certain threshold (long-day plants) while others bloom only when the day length drops below a certain threshold (short-day plants). Tropical plants, including most aquarium plants, would be expected to be day-neutral, and in any case we rarely grow them for their blossoms. I have seen claims that aquarium plants cannot sustain photosynthesis for more than about eight hours before needing a rest, but I can find no confirmation of this in scientific sources. However, it's certainly true that a short day length hinders plant growth and it seems plausible that a long day length may favor algae over green plants. The safest bet seems to be to give aquarium plants a day length comparable to that which they are accustomed to in nature.
Alas, there is no simple answer. There are so many variables that this is a matter of art, not science. But science can at least inform our art.
It seems best to stick with a photoperiod of 8 to 10 hours, similar to what plants experience in tropical settings. This can be done manually or using a timer. If you have a long work day, as I do, you may set the timer to split the day into two photoperiods so that the tank will be illuminated for your enjoyment when you are home in the mornings and evenings. This has worked well for me and other aquarium keepers.
It is much harder to give a good answer on how much light and of
what type should be provided. Light requirements vary greatly from
dim light plants, such as Java moss, to very bright light plants,
such as cabomba. Very roughly speaking, cabomba requires about
three times as much light as Java moss to be at its best. For
plants requiring fairly bright conditions, without carbon dioxide
injection, and with adequate nutrients, an illumination of at
least 1000 lux is desirable, assuming a light quality typical of
fluorescent tubes. This corresponds to a PAR of about 15
micromoles/m2/s. You will see recommendations for much
higher PAR at planted aquarium discussion sites. These assume that
carbon dioxide is being injected.
To determine lux, one must know the area over which a given lumen of lighting will be spread. For example, if the light source gives 1700 lumens, and will be spread over about a square meter of tank floor, the illumination will be 1700 lux. This assumes that the light is properly directed downwards; that the aquarium cover, usually located between the light strip and the water surface, is not reflecting or absorbing a significant amount of light; and that the water is clear, so that it also does not absorb a significant amount of light.
The greatest uncertainty is in how much light the plants can
usefully use. If nutrients and carbon dioxide are scarce, the
plants cannot use bright light over a long photoperiod. There are
simply not enough nutrients available to sustain growth.
Conditions of bright light and low carbon dioxide are widely
recognized as favoring the growth of black brush algae, which
competes effectively with plants for nutrients under these
conditions. One of the reasons for carbon dioxide injection is to
promote rapid plant growth under bright lighting, and the brighter
the lighting, the more benefit comes from carbon dioxide injection
and fertilization. This means that the aquarium keeper must
closely monitor his tank to determine if his light level is in
balance with nutrients, and make adjustments. The adjustments may
well be to the rate of fertilization and carbon dioxide injection
rather than to lighting levels.
Inadequate light for the available nutrients results in etiolation,
where leaves are small, yellowed, and (in stem plants) the
distance between leaves on the stem becomes greater. Leaves lower
on the stem die and drop off. Unfortunately, lack of nitrogen has
a similar effect, but it is possible to monitor nitrate levels and
verify that nitrate is not deficient.
Excessive light results in bleaching, where chlorophyll is destroyed because it absorbs light energy faster than it can use it. Bleaching is characterized by leaves of normal form and spacing that are nonetheless yellowish and may develop dead patches. However, it is almost impossible to achieve light levels in a home aquarium that result in bleaching, except for very sensitive dim-light plants such as ceratophyllum (hornwort). The worst that can happen in an aquarium with ample nutrients is that the light is wasted. However, where light levels are high in relation to nutrients, there is often a characteristic pattern of faster-growing plants flourishing while slower-growing plants languish or deteriorate. With ample light, the faster-growing plants out-compete the slower-growing plants for nutrients, which starves the slow-growing plants.
My impression is that complete aquarium kits for 10 to 37 gallon aquariums come with lighting adequate for plants with moderate light requirements without carbon dioxide injection. These are what some aquarium keepers describe as "low-tech tanks." For "high-tech tanks" featuring heavy planting with carbon dioxide injection and regular fertilization, the aquarium keeper is better off to avoid complete aquarium kits and purchase lighting and other components independently.
*There
is, alas, to my knowledge, no Hobbes cycle.
Next: Unwanted guests
Copyright ©2019 Kent G. Budge. All rights reserved.