

When we set up an aquarium, we seek to produce a warm, chemically
benign, nutritient-rich environment in which our plants and fish
thrive. Such a favorable environment is attractive to uninvited
guests. At best, these guests merely compete with our regular
aquarium inhabitants for nutrients, and may even be beneficial. At
worst, they regard our regular tank inhabitants as
nutrients. Some can wipe out a previously healthy tank in short
order.
Scientists love classifying things, because it is a way to bring order to their understanding of the beautiful variety of nature. Biologists are particularly fond of classification, and they broadly classify all interactions between living things as short-term or long-term. Short-term interactions include predation and pollination; only the former is of much interest to aquarium keepers. A clown loach feeding on a snail is a predator. For our purposes, so is a clown loach feeding on a prized Amazon sword plant.
Long-term interactions, or symbioses (singular symbiosis), are classified by whether one or both species involved are helped, harmed, or largely unaffected by the relationship. This can be diagrammed.
Aquarium keepers are undestandably less interested in the welfare of uninvited guests than of their regular tank inhabitants. They are also less interested in relationships that are largely neutral with respect to their regular inhabitants. This means that mutualism and commensalism, where our regular aquarium inhabitants benefit from the presence of guests, and parasitism, amensalism and competition, where our regular aquarium inhabitants are made worse off by uninvited guests, are of greatest interest.
I'll therefore look at uninvited guests from the perspective of whether they are predators on our regular inhabitants, are parasites on our regular inhabitants, are competitive pests, are harmless, or are actually beneficial. Predators and parasites are two of the relationships formally recognized by biologists. Pests are either amensals or competitors, depending on whether the guest is significantly hamred by the regular tank inhabitants. Harmless guests are mostly neutral or commensal, depending on whether the uninvited guest gains significant benefits from being around the regular inhabitants. Beneficial guests are either commensal or mutualistic, depending on whether the guest benefits significantly from the presence of our regular aquarium inhabitants.
Much of what I present here is purely for its scientific
interest. However, there is obvious practical value in knowing how
to rid our aquariums of predators, parasites, and pests, and how
to encourage beneficial guests to flourish.
The regular inhabitants of most planted aquariums come from just a few branches of the tree of life. Most fish are from a single branch (Actinopterygii) of the chordates (vertebrates and their close kin), which are a branch of bilaterians (animals with bilateral body plans), which are a branch of the animal kingdom, which falls within domain Eukaryota. Snails are gastropod molluscs and shrimp are crustacean arthropods, which are also bilaterians. A few planted aquariums also include amphibians, which are relatively close relatives of fish, and crabs, close relatives of shrimp. Our plants are mostly tracheophytes (vascular plants), though we keep some mosses (bryophytes) and (less commonly) red or green algae.
Uninvited guests range much more widely across the tree of life.
This is now known to be much more complicated than the ancient
division into animals and plants. Biologists recognized that many
microscopic organisms were neither truly animal or vegetable,
based on their cellular structures, even before we gained the
ability to sequence the entire genome of different species.
Genetic sequencing has confirmed suspected relationships, and
uncovered unsuspected relationships, by revealing common genes
between different species.
The more fundamental a gene is to carrying out life processes,
the more likely it is to have settled into its current form early
in the history of life, and the less likely it is to have changed
much since then. Such gene sequences are described as highly
conserved. Arguably the most fundamental of all genes are
those that dictate how genes themselves are translated into
proteins. These include ribosomal RNA, which makes up the
individual protein factories. These are very highly conserved.
Biologists confirmed soon after rapid sequencing became possible
that most bacteria have very similar ribosomal RNA, but it is
significantly different among what where then called
archaebacteria, which more closely resembled ribosomal RNA of
eukaryotes. This led to the recognition of the three main
divisions or domains of life, consisting of the bacteria,
the archaea, and the eukaryotes.
Bacteria are very simple single-celled organisms in which, for the most part, everything takes place in a single cellular compartment. Archaea resemble bacteria (and were long classified as such) but have very distinctive biochemistry that is actually closer to that of the eukaryotes. Eukaryotes have more complicated cells with multiple compartments (including, at a minimum, a nucleus to hold genetic information) and include plants, fungi, animals, and ourselves, as well as complex single-celled forms of life that are able to move about, traditionally identified as protozoa, and simple photosynthetic organisms, identified as algae. Although the protozoa and algae are now understood to include many groups that are not closely related, and there is significant overlap between the two groups, these terms are still in common use among aquarium keepers and others.
Here is a simplified diagram of the tree of life as currently
understood. I have eliminated some levels that are still
controversial or do not distinguish living organisms of interest
to aquarium keepers, and branches that do not include common
aquarium inhabitants. Click to enlarge.
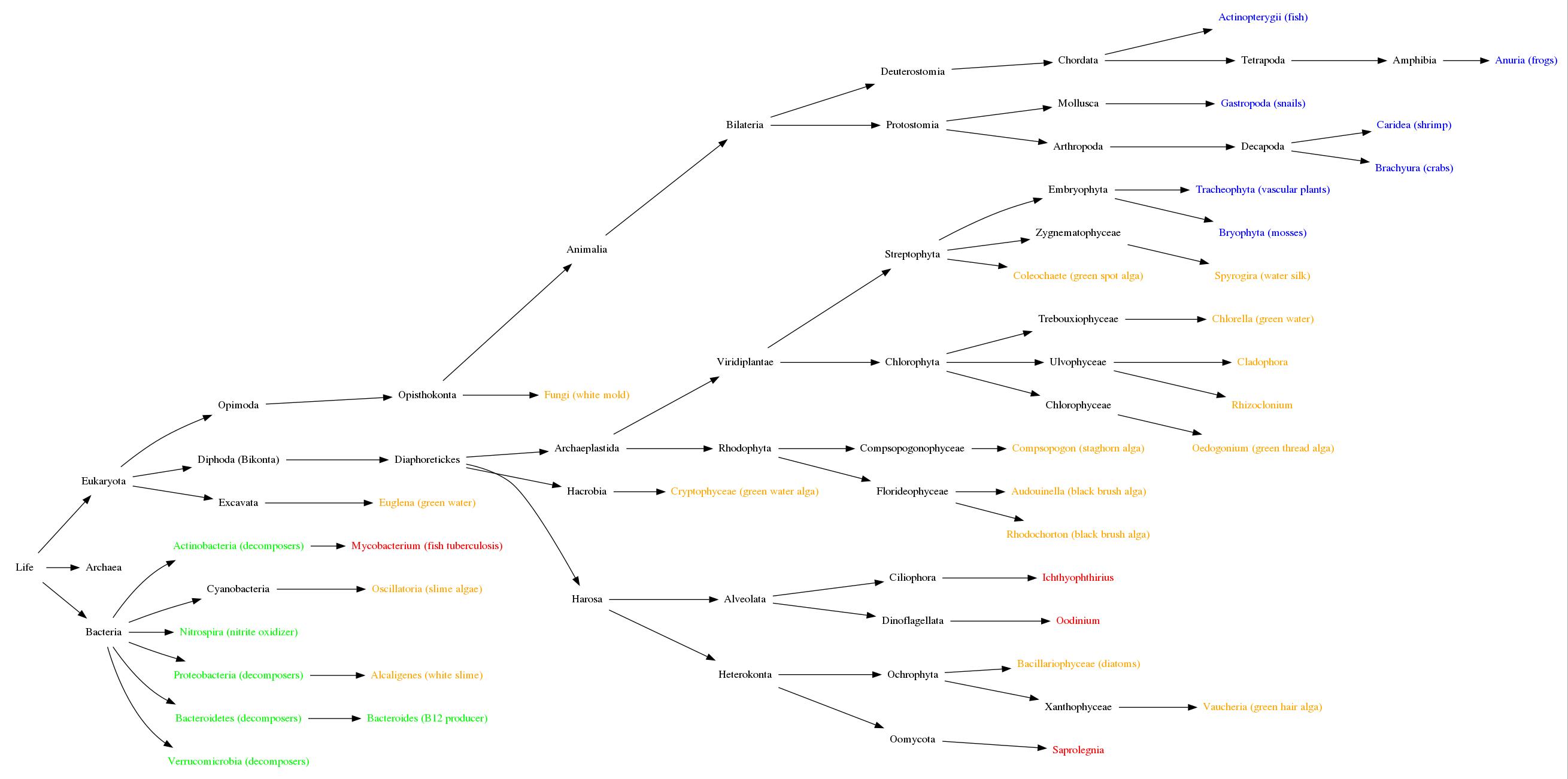
Regular tank inhabitants are colored blue; welcome guests are
green; pests are yellow; and parasites or predators are red.
Predators are guests whose short-term interaction with our
regular tank inhabitants is to kill and eat them. Many of these
are not actually uninvited, but are organisms we have deliberately
introduced into our tanks. We then discover to our sorrow that
they have made themselves unwelcome by developing a taste for our
other fish, invertebrates, or plants.
It's really no surprise that certain species of fish are not good choices for a planted aquarium. The following tropical fish have bad reputations:
All of these either have a tendency to chew up plants, or are
burrowers that uproot them. An additional difficulty with
plecostomus and clown loaches is that, under ideal conditions,
they can grow to be very large, more than many novice aquarium
keepers realize.
Fish that seem well suited to planted aquariums include most other tetras, gouramis, rainbowfish, livebearers, and rasboras.
Parasites are organisms that satisfy their nutritional needs by attaching themselves to a larger organisms and feasting on its tissues. They are distinct from predators, which prey on smaller organisms that are typically eaten whole, and from mutualists, which "pay rent" for taking nutrients from a larger organism by giving important benefits in return. A clown loach feasting on a snail is a predator; a nitrogen-fixing bacteria living in a plant root is a mutualist; and a ciliate eating into the skin of a fish is a parasite.
Bacteria are organisms composed of single cells or simple
groupings of identical cells. Their cells are very simple,
consisting of a single compartment enclosed by a membrane and cell
wall. Their library of genetic blueprints consists of a single
loop of DNA more or less floating free within the cell. Some
bacteria have additional smaller loops of DNA called plasmids
(not to be confused with plastids like chloroplasts or
mitochondria) that carry genes for specific enzymes and which can
be transferred from cell to cell via a primitive form of sexual
conjugation.
Most bacteria are less than a micrometer in size, putting them at
the very limit of what can be seen with a light microscope. Most
are also colorless, so that they barely stand out from the
background. As a result, pathologists trying to identify
infections must be able to quickly narrow down the possibilities
based on simple criteria. Thus bacteria are roughly classified by
shape, staining characteristics, and oxygen sensitivity.
Typical bacterial shapes are spheres (sometimes called cocci),
rods (sometimes called bacilli), or helixes (sometimes
called spirochetes, though this specifically refers to
spiral bacteria that are very thin and long.) Bacteria with
similar shapes are not necessarily related, but shape is useful in
combination with other characteristics for identifying a
bacterium.
Staining characteristics are determined by the Gram staining
procedure, in which crystal violet, which is dark purple in color,
is used to stain a sample on a microscope slide. This is then
leached with alcohol and counterstained with a red dye.
Gram-positive bacteria have a thick cell wall that retains the
dark purple dye, while Gram-negative bacteria have a thinner wall
that does not, and these appear red due to the counterstain. Some
bacteria do not really fall into either category; these include
acid-fast bacteria, which have a waxy cell wall that retains
carbol fuchsin stain even after brief boiling in acid. Bacteria in
each group tend to be related to each other, but not necessarily
closely, and there are Gram-indeterminate bacteria that stain
differently when grown under different conditions that complicate
things.
Oxygen sensitivity breaks down into obligate aerobes
(often just aerobes), which require oxygen for growth; obligate
anaerobes (often just anaerobes) that are harmed by
oxygen; facultative anaerobes, which will use oxygen when
it is available but don't require it; microaerophiles,
which requires small amounts of oxygen but are harmed by
atmospheric concentrations; and aerotoloerant organisms
which do not use oxygen but are not harmed by it. Oxygen
sensitivity is assessed by placing a sample in a test tube
containing a culture medium impregnated with thioglycolate, which
reacts with oxygen to remove it from the medium. Oxygen diffuses
back into the medium at the top of the tube but remains absent at
the bottom, and oxygen sensitivity can be determined by where the
bacteria congregate and multiply.
Describing a bacterium as, for example, an aerobic Gram-negative
rod immediately narrows down its possible identity. More precise
identification requires identifying what substances it can feed
on, with exact identification requiring genetic sequencing. In a
hospital setting, form and staining characteristics are important
for making a quick informed guess, culturing takes more time but
is more sure, and sequencing is used only for especially dangerous
infections that require precise identification. In many cases, serology
is used to quickly identify specific strains of bacteria. This
looks for a reaction of specific antibodies collected from human
or animal blood serum with the bacterium.
Classifying bacteria is further complicated by their plasmids. Because these can be transferred from bacterium to bacterium, a process called horizontal transfer, it is very difficult to work out the evolutionary relationships of bacteria based purely on their outward characteristics. Some bacteria long regarded as different species have now been recognized to be essentially the same species with or without a particular plasmid, and since a plasmid can be transferred from one to another, this would imply that a bacterium can change its species!
An additional classification criterion, less used in medical work
but of interest to researchers, is motility. Many bacteria have
one or more flagella, long whiplike organelles that propel
them through the water. These are motile bacteria that
stand out particularly in dark-field microscopy, where the culture
is lit from the side and tiny particles show up as bright specks
(like dust motes in a sunbeam). Bacteria lacking flagella may move
slowly across a surface by gliding, while many other
bacteria are nonmotile.
Here we are interested in bacteria in a fish tank, where we will usually have to do without precise identification when we suspect a bacterial species is causing trouble.

Columnaris in a chinook salmon.
Via Wikimedia
Commons.
Columnaris, sometimes called "cotton mouth", is caused by Flavobacterium columnare, a thin Gram-negative rod. It is a member of a bacterial phylum called Bacteroidetes. Most are harmless or even beneficial, but columnaris is a serious opportunistic parasite of fish. Opportunistic parasites are organisms that normally are free living, but will take advantage of the opportunity to attack another organism that is already weakened and has a reduced ability to resist infection. Columnaris manifests as pale spots around the head, on the fins, or around the gills. These spread down the fish, particularly along its back, and the lesions around the mouth develop visible filaments that give the disease its common name.
Prevention is by keeping a generally clean tank and feeding fish a good diet. Sick or injured fish should be quarantined. Spread of the organism between tanks is avoided by disinfection of nets and other implements.
Treatment is with copper or antibiotics. Only antibiotics are much
use against an internal infection. The most effective seems to be
the tetracyclines, though acriflavine and furan
can also be effective.
These acid-fast aerobic rods are from the same genus as the bacteria that cause tuberculosis and leprosy in humans. There are several species that can cause a disease called fish tuberculosis. This typically causes its victims to slowly waste away, or to accumulate fluids in their bellies (dropsy) so that they swell up. Either way, it's a slow and unpleasant death for the fish. The human version of these diseases requires a long course of antibiotics to be cured. This is impractical for fish, which are best euthanized if they show signs of this infection. It can spread to other fish, especially when they cannibalize their dead companions. More frightening is that it can spread to aquarium keepers who reach into an aquarium when they have an open wound or sore on their hand. Fortunately, the disease is not terribly common.
This is a very large phylum of bacteria, typically further broken
into categories designed by Greek letters (alpha, beta, gamma ...
proteobacteria.) They all have relatively thin cell walls
(Gram-negative) and have very similar ribosomal RNA sequences.
Otherwise, they are a very diverse group, so variable in form that
they were named after Proteus, a Greek god of the sea capable of
taking on many shapes.
Most are harmless or even beneficial in the aquarium. However, some are opportunistic pathogens.
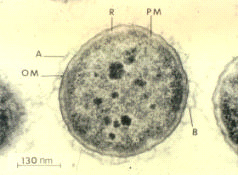
Micrograph of A. salmonicida. Via Wikimedia
Commons.
Aeromonas is a genus of gammaproteobacteria that causes a lot of problems for koi ponds or salmon farms. It is a Gram-negative facultatively anaerobic coccus. It mostly attacks salmonids and cyprinids (carp), which are not common inhabitants of a planted aquarium, but it can occasionally cause problems for the planted aquarium keeper. As with many other bacteria, it is mostly an opportunistic pathogen, and maintaining a tank in good condition is the best preventative. If it does get loose in the tank, it manifests as bloody sores on the skin of the fish. It can be treated with antibiotics effective against Gram-negative bacteria.
Pseudomonas are close relatives of Aeromonas and
most of the same observations apply. However, it differs in being
a motile aerobic rod. It is almost entirely an opportunistic
pathogen, harmless until a fish is already in bad shape, and then
causing the symptoms of fin rot. Fin rot is not a definitive
diagnosis; it is a symptom that can be caused by many pathogens in
fish that are already in a very bad way. Pseudomonas
fluorescens is the most likely culprit, being a common agent
of fin rot in very badly stressed betta fish.
An aside: I like betta fish very much; they are among the first
tropical fish I ever kept. I have a betta now, kept in a
carefully maintained 5-gallon tank with a snail companion. But the
aggressive behavior of males almost mandates that betta be sold in
those ridiculous little cups you see in fish stores, and a betta
in a little cup of water is always going to be stressed. If you
can find a local fish shop that places individual betta males in
larger tanks with other species (bettas are rarely aggressive
except towards other labyrinth fish) then that is the shop at
which to buy your betta. If you can't find such a shop, it may
actually be more humane to purchase one at a big box pet franchise
with high turnover, so that no individual betta stays in the cup
on the shelf for long.
The great majority of protozoa in our aquariums, though
uninvited, are not unwelcome. They are the microscopic
counterparts of snails and algae-eating fish, feasting on
overgrowths of bacteria and algae and thereby keeping them in
check. Only a few species are parasitic on plants or fish.
Unfortunately, these can cause a lot of trouble.

Perhaps the the worst of the parasitic protozoa is Ichthyophthirius multifiliis, "fish louse with many children." This is a ciliate, a member of a large phylum of protozoa whose most distinctive trait is the presence of cilia. These are hairlike organs that move in a coordinated way to propel the ciliate through the water or to draw water past the ciliate. Other characteristics shared by most cilia are a mouthlike organelle called a cytostome, through which the ciliate takes in food particles (typically smaller microorganisms), and a division of its genetic material between a macronucleus and a micronucleus. The micronucleus is the ultimate set of blueprints for the ciliate, and it is duplicated during asexual reproduction and swaps genes with another ciliate during sexual reproduction. The DNA blueprints in the micronucleus are copied numerous times in the macronucleus, which is where DNA is copied to RNA that will then serve as the template for protein synthesis.
Most ciliates are harmless or even beneficial, but a few species
are parasitic, and ich (as we call it for short) is one of the
worst. Most experienced aquarium keepers have had to deal
with one or more bouts of ich. Fortunately, it can be prevented by
appropriate quarantine, and an
infestation that gets through quarantine can be eradicated with
suitable chemical treatment.
Ich is the fish equivalent of smallpox. It covers the fish with
small white spots (about the size of a grain of salt) but the
worst damage is inside the gills, where it is not as visible. Fish
heavily infested with ich die from oxygen starvation from the
smothering effect of the gill infestation, or from loss of
electrolytes through their damaged skins. Ich has no other
host than fish and does not survive long on its own, but it
spreads rapidly and kills most of its victims if left untreated.
Fish that survive ich develop significant resistance to future
infestations from the same strain, but a practical ich vaccine for
fish has yet to be developed.
Ich has a fairly elaborate life cycle. The spots you see on your
fish are the feeding stage of the parasite, known as the trophont.
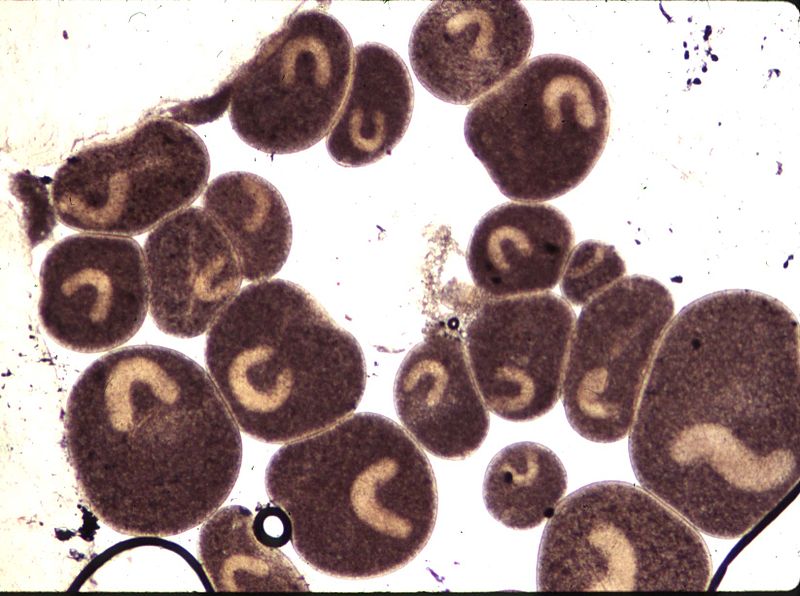
The trophont is a single large cell, covered with a coat of cilia and with a very large horseshoe-shaped macronucleus. The white spot consists both of the trophont and of the inflamed tissues of the fish that surround it. By the time a white spot is visible, the trophont has already been feasting on the fish for some time and is close to maturity.
A mature trophont is up to a millimeter in size. When it reaches
maturity, it burrows back out of the fish and drops to the bottom
of the tank, where it transforms itself into a tomont. A
tomont is sometimes described as a cyst, but it it not a true
protozoan cyst: It cannot survive being dried out, and it cannot
remain dormant for more than a short time. A tomont is sticky and
will adhere to whatever solid object it comes in contact with,
including a fish net dragged through the aquarium. It is a
reproductive phase of the organism, in which the giant cell
divides into several hundred therodonts, also known as tomites.
Once the tomont has finished dividing into therodonts, the therodonts burrow out of the tomont wall and become free-swimming organisms. These resemble other ciliates but apparently have none of the usual organelles for capturing and digesting smaller microorganisms. They must find a host fish within about 12 hours or they will starve. Those that find a host burrow into its skin or gill membranes, transform into juvenile trophonts, and begin feasting on skin and blood cells.
This life cycle explains why ich is so explosively dangerous and why it must be treated the way it is. The complete cycle from trophont to trophont takes about seven days at a typical tank temperature of 25 C (77 F). It is much slower at colder temperatures (eight weeks at 6 C (43 F) and faster at higher temperatures. Above 30 C (86 F), the tomonts of most wild strains are unable to replicate at all, but it is now clear that heat-resistant strains have evolved at fisheries that can withstand temperatures higher than the fish themselves can long tolerate. Each tomont produces hundreds of offspring, so a single infested fish can spread the infestation throughout the aquarium in just a few days.
The first symptoms of ich are often behavioral, appearing even before the first white spots are noticeable. These behavioral clues are likely due both to initial infestation of the gills (which is not outwardly visible) and to the fish's reaction to infestation, which is similar to the general feeling of malaise (prodrome) we experience at the start of a bad bout of influenza or other infectious disease. Infested fish will often gather at the surface of the water, sometimes over the heater or in another warm part of the tank, and become listless and lose interest in food. Close inspection will then reveal a few characteristic white spots. Fish may "flash", that is, scrape themselves on rocks or other sharp surfaces in the tank, apparently in an attempt to dislodge the parasites. Some species of fish, such as Siamese algae eaters, will occasionally flash even when healthy, but any increase in flashing should be considered a warning sign and the fish should be closely inspected.
The definitive diagnosis at commercial fisheries is made by netting out one of the fish and taking a scraping of its skin to search for trophonts under the microscope. Home aquarium keepers may not have a microscope and are understandably loath to stress an already sick fish in this manner, and, for us, the appearance of white spots the size of salt grains on the fish is considered definitive. At this point, the fish are already heavily infested and likely to die if treatment is not undertaken immediately.
The trophont and tomont stages of the ich life cycle have considerable resistance to any treatment that will not kill the fish as well. As a result, all treatment approaches other than heat treatment are aimed against the free-swimming therodonts. If these can be exterminated, the life cycle is broken. With no new organisms burrowing into their skins and gills, the fish can begin to heal as the tomonts drop off, encyst, and produce therodonts that are destroyed by treatment as fast as they emerge.
Heat treatment was long a standby of aquarium keepers who were keeping fish able to withstand the high temperatures required (30 C or 86 F). For example, discus keepers often kept their fish permanently at 86F and almost never experienced an ich infestation. Unfortunately, there are now credible reports, supported by my own experience, of strains of ich that can withstand such temperatures and cannot be eradicated by heat treatment alone. Nevertheless, the first step in treating ich is almost always to raise the tank temperature as high as the fish can comfortably stand for a couple of weeks. This will be somewhere in the ballpark of 28C (82 F) for most tropical fish. The idea is to speed up the ich life cycle, which makes treatment more effective by pushing the organisms into the therodont stage that is vulnerable to medications.
The first line of treatment is malachite green and formaldehyde. Other treatment options are methylene blue, quinine, and salt baths. Keep in mind that if a single fish shows signs of ich, the entire aquarium must be regarded as infested. Moving the entire fish population of a display aquarium into a hospital tank is not practical for most of us, though it is an excellent idea if it is. Treatment then requires less medication, and more treatment options are open. The display tank need not be medicated if every fish is removed, since the infestation will be starved out after a few days. The hospital tank must not only have enough room for the entire stock of fish, but it must also be cycled adequately for such a large stock.
Treatment is complete when the fish return to their normal behavior and show no white spots for at least three days.
Prevention is vastly better than treatment. Ich has no host other than fish and it has no true carrier state. Thus ich can only come in to our aquariums as an unwelcome hitchhiker on plants or animals added to the tank.
There are three approaches to ensuring plants do not bring ich tomonts into an aquarium. The first is to obtain plants from sources we can be certain are free of ich. This generally means plants grown in the absence of any fish whatsoever. This will include the increasingly popular tissue cultured aquarium plants. The second approach is to disinfect plants obtained from unreliable sources. The third approach is quarantine.
Quarantine of
fish is the only reliable way to prevent new fish spreading ich
to the display tank. Even the best fish shops are constantly
bringing in fish from supply chains that are constantly
struggling to control ich infestation, and it is all too easy to
purchase a fish that has already been infected but does not yet
show symptoms. I personally resolved to never skip quarantine
after battling two ich infestations in one year.

Oodinium is remarkably similar to ich in its symptoms and life cycle, in spite of being only distantly related. It is a dinoflagellate, a member of a family of single-cell organisms traditionally regarded as algae. Modern molecular biology puts the dinoflagellates in the Alveolata, a clade (branch of the tree of life) alongside such unlikely relatives as ciliates and the malaria parasite, and distinct from other algae.
Dinoflagellates have a pair of flagella and a distinctive set of
outer membranes. Most are photosynthetic, but most also consume
smaller microorganisms. Their chloroplasts are unusual in having
an additional outer membrane, which suggests their origin was as
an algal symbiote rather than a cyanobacterium. Most are
free-living marine organisms, though some live in fresh water.
Oodinium (actually Piscinoodinium pillulare) has
chloroplasts and carries out photosynthesis, but it also attaches
to the skin of fish and feeds on their skin and blood cells. Like
ich, it has a life cycle consisting of a reproductive palmella, a
host-seeking dinospore, and a parasitic trophont. The trophonts
are much smaller than ick and have a brownish to gold color, so
that fish appear to be covered with brown velvet. This gives the
parasite its common name of velvet disease.
The condition seems to be less common than ich but is no less
dangerous.
Fish will show the same behavioral symptoms as for ich. The trophonts are tiny and are best seen by dimming the room lights and shining a flashlight directly on the fish. This will show an iridescent golden film on the fish.
The same treatment protocols are effective for oodinium as for ich. There is some tendency for aquarium keepers to use copper, if possible, for treatment, since Oodinium is very sensitive to copper. However, malachite green plus formalin can also be effective, and it is much less toxic to invertebrates. Salt baths are also effective when practical; the trophonts will reportedly actually detach from the fish in saline water and can be rinsed away. Acriflavine, which is less commonly used for ich, is effective against oodinium.
The chief distinction in treatment is that oodinium gets significant nutrients via photosynthesis. The infestation can thus be slowed down and more easily eradicated if the tank is kept as dim as possible.
Oomycota are sometimes described as "water mold", and colonies do
have a mold-like appearance. However, they are not fungi. An
oomycote was responsible for the devastating Irish potato famine
of the 19th century. Oomycotes are part of a large group called
Harosa or Sar, which also includes diatoms, Oodinium, and
Ichthyophthirius. The Harosa and the Archaeplastida, the
red and green algae and green plants, plus some other small
groups, make up the bikonts, which are all thought to be
descendants of a single eukaryotic cell with two flagella. The
Harosa is a very diverse group, which includes both photosynthetic
and non-photosynthetic organisms.
Oomycota can be distinguished from most true fungi under a
microscope by the absence of any cross-walls in their threadlike
bodies. Their cells walls also do not contain chitin, the
characteristic cell wall material of fungi, but this is not
something the home aquarist is likely able to determine. A good
rule of thumb is that most threadlike infections of fish or their
eggs blamed on "fungus" are actually oomycotes.
Oomycotes are nonphotosynthetic and are mostly decomposers, but
some are opportunistic pathogens.
Saprolegnia rarely attacks a healthy animal. However, if a fish
or amphibian is stressed, injured, or already diseased,
saprolegnia can take hold and quickly finish off the animal.
Saprolegnia also spreads quickly from dead eggs to neighboring,
previously healthy, eggs. Young fish, with less mature immune
systems, are also particularly vulnerable. The disease spreads as
flagellated zoospores.
Prevention is by maintaining a generally healthy tank and by quarantining sick or injured fish. Infection is more likely in cooler water, giving this parasite yet another common name, "winter kill." Treatment is normally based on malachite green, as for ich. Be sure it is saprolegnia and not columnaris; the latter requires antibiotic treatment that is not much use against Saprolegnia.
These are a genus of oomycotes that attack gills, producing a
condition called gill rot. The fish show the usual signs of gill
infection, namely, gathering close to the water surface and
gasping. If you catch a fish and open its gill covers, you will
see the lesions on the gills.
Like Saprolegnia, Brachiomyces is mostly an opportunistic pathogen, and maintaining a healthy tank is an excellent preventative. Quarantine of sick fish and prompt removal of dead fish is imperative. An infected fish does not have a good chance of being saved, but treatment with malachite green offers some hope. Banchiomyces is more likely to spread to other fish than Saprolegnia, so it may be prudent to treat the entire aquarium with malachite green. Fortunately, gill rot is not a common pest.
This was long thought to be a fungus, since it forms threadlike
colonies that have chitin in their cell walls, like fungi do.
However, genetic sequencing has shown that it is more closely
related to animals. It produces skin lesions on fish, but the real
damage is internal, resembling fish tuberculosis. Infected fish
show an odd rocking motion, giving the disease its common name,
swinging disease, due to damage to their central nervous systems.
It can spread from fish to fish and prevention includes promptly
removing any infected fish from an aquarium. There is no known
treatment, and affected fish are best promptly euthanized.
Fungi are a kingdom of organisms traditionally regarded as plants
but now known to be much more closely related to animals. Both
animals and fungi are opisthokonts, thought to be
descended from a ancient unicellular organism propelled through
water by a single rear flagellium. However, most fungi have lost
their flagella, and fungal cells are surrounded by a wall of chitin,
a polymer formed from glucosamine. Most fungi are benign or even
beneficial in a fish tank, since they are able to break down some
components of dead organic matter (particularly cellulose) that
other organisms cannot.
Fungi get a bad rap with aquarium keepers. Oomyces such as Saprolegnia and Branchiomyces are often described as fungus, but they are not even close fungus relatives. Ichthyophonus is more closely related to fungi, but is now considered a closer relative of the animal kingdom.
Pests are free-living organisms that are unattractive, compete
for nutrients, and can overrun the tank. They are distinct from
parasites and predators in that they do not directly feed on our
regular aquarium inhabitants.
Oscillatoria is a genus of cyanobacteria, sometimes known
to aquarists as slime algae or blue-green algae. Cyanobacteria are
bacteria capable of producing oxygen by photosynthesis. They are
believed to have descended from a single ancient bacterium that
figured out the trick of generating oxygen through photosynthesis
without poisoning itself. All chloroplasts within eukaryotic
photosynthesizing organisms are also believed to be descendants of
this ancient bacterium. Cyanobacteria look a lot like a
chloroplast (or vice versa), with deep folding on an inner
membrane to produce a large surface area on which to conduct
photosynthesis. They differ from the chloroplasts of green plants
mostly in having phicobilisomes, structures that contain
pigments capable of absorbing light at wavelengths that
chlorophyll does not absorb well, and passing its energy to the
chlorophyll of Photosystem II. In contrast with green plants and
green algae, the chloroplasts of red algae have retained their
phicobilisomes.
Oscillatoria are capable of gliding movement, and seeing the
threads wave slowly back and forth under the microscope is
slightly unsettling. They are also capable of reducing atmospheric
nitrogen to supply their nitrogen needs.
Oscillatoria is a normal inhabitant of aquariums, but when it gets out of control, it can form a malodorous bluish-green film that creeps across hardware, glass, and even plants and substrate. They have a competitive advantage over aquarium plants in an environment rich in yellow-green light, with abundant phosphate but limited nitrate. Because almost all bacteria are flexible in their nutritional requirements, cyanobacteria may also thrive on excess dissolved organic carbon in the tank. However, most oscillatoria blooms are likely to be the result of a lack of the usual protozoa that feed on filamentous cyanobacteria. They are thus a particular risk in an aquarium that has been treated for protozoa parasites such as ich.
Oscillatoria do not hold fast to surfaces, so minor infestations
can be vacuumed away during water changes. Heavier or recurring
infestations can be controlled by adjusting lighting and making
sure there is adequate nitrogen, and by introducing substrate or
filter sludge from a healthy aquarium to inoculate the infested
aquarium with predatory protozoa.
If these measures are inadequate, it is possible to eradicate oscillatoria by blacking out the aquarium. Turn off all lighting and put dark paper or cloth around the top and sides of the aquarium to remove light. Oscillatoria requires light for photosynthesis, so excluding light will stop its growth dead in its tracks. Predatory microbes should then be able to consume the resting oscillatoria and eliminate it from the tank. Green plants should be able to endure a significantly longer period of darkness than cyanobacteria, but do not exclude light longer than necessary.
Another approach that has been recommended is to raise the potassium concentration in the tank to 30 ppm. This seems to inhibit cyanobacteria without doing any harm to green plants.
A last-resort treatment is to dose the tank with either copper or an antibiotic.
The National Institutes for Health have actually tested
antibiotics against cyanobacteria. Of those antibiotics commonly
used by aquarium keepers, they find that amoxicillin is most
effective, followed by tetracycline
and kanamycin. Of these, NIH did not test erythromycin, which is
sold under various brand names as a fish antibiotic and is claimed
to be effective against cyanobacteria. All seem likely to damage
the biological filter in the aquarium, so this should be removed
and stored in well-aerated water during antibiotic treatment.
Many things can cause the water in an aquarium to cloud up, including purely inorganic causes such as adding poorly washed substrate or allowing calcium levels to become high enough at high pH to precipitate calcium phosphate or calcium carbonate. However, cloudy water can also be caused by a bacterial bloom. The causative organism can come from almost any branch of the bacteria, but it is also almost always self-limiting. Protozoa will multiply and feast on the bacteria until they are brought under control.
This is a billowy mass of almost invisible white threads that frequently forms on fresh driftwood added to an aquarium.

The exact identity of the filamentary white growth is unknown. It may even be a fungus. However, a similar infestation seen in saltwater aquariums is attributed to Alcaligenes faecalis, a betaproteobacterium. It is a Gram-negative aerobic rod which is a very common and highly adaptable bacterium found in many environments. It thrives on organic compounds leaching out of the driftwood.
However, growths like this seem to be harmless and self-limiting, usually disappearing after a few days when the food supply is exhausted. White slime can easily be vacuumed up during a water change. It can be avoided by thoroughly leaching the driftwood before placing it in the aquarium.
Algae are a diverse group of single-celled and colonial organisms
that carry out photosynthesis. Only the green algae are closely
related to green plants. They are normal tank inhabitants but
become unsightly if their growth goes out of control and they take
over the aquarium. Dealing with algae is less a case of
eradicating them than of keeping them in check.
Aquarium keepers tend to identify algae by their form as seen
with the naked eye. This is understandable, but some forms of
growth are characteristic of algae from very different branches of
the tree of life. Thus, terms like "green thread algae", "fuzz
algae", and "hair algae" are descriptive rather than taxonomic
terms, and algae with these forms are found on scattered branches
of the tree of life.
Achnanthes |
Didymosphenia |
Diatoms, Bacillariophyceae, are single-celled algae that are not closely related to green plants or most other algae. They are harosa, like Saprolegnia, Oodinium, and Ichthyophthirius
Most of the photosynthetic Harosa are grouped as the Ochrophyta.
They are thought to be descendants of a single eukaryotic cell
with two unequal flagella, which engulfed a red alga cell and,
with it, its chloroplast. This is described as secondary
emdosymbiosis, since the chloroplast was a primary
endosymbiote within a red alga that itself became an endosymbiote.
The evidence for this is that their chloroplasts are surrounded by
four membranes rather than the two membranes of chloroplasts in
red and green algae and green plants.
The distinctive feature of diatoms is that they surround themselves with a silica shell. This is beautiful under a microscope. Diatoms are an important part of many ecosystems, and their photosynthesis produces 20% to 50% of the Earth's oxygen production via photosynthesis. There are both freshwater and marine diatoms.
Diatoms often appear in new aquariums as a brown film or crust that feels gritty to the touch but is fairly easily wiped away. Diatoms thrive on the silica from fresh substrate and in the lack of competition for nutrients. They almost always disappear in time, in part because of competition for nutrients from plants and other algae, and in part because they are feasted on by everything from algae-eating fish to microscopic amoebas. When diatoms persist in a mature tank, this is typically an indication of high levels of silica in the water supply. Silica can be diluted with deionized water or removed by ion exchange, but ion exchange media that remove silica tend to remove phosphate needed for plant growth as well. Because their chloroplasts are essentially red algae chloroplasts, diatoms thrive on the yellow-green light that green plants absorb poorly, so the use of grow lights will help plants compete with diatoms.
Red algae (Rhodophyta), together with green algae and
green plants, are archaeplastids. Archaeplastids are
thought to be descendants of a single ancient eukaryotic cell
bearing two flagella (a bikont) that engulfed a
cyanobacteria. This cyanobacteria took up permanent residence and
became a chloroplast.
The red algae are a branch of the archaeplastids whose chloroplasts retained the phycobilisomes of their blue-green algal ancestor, but which lost the flagella of the eukaryotic ancestor. Phycobilisomes contain photosynthetic pigments that absorb light at wavelengths that chlorophyll does not, which they channel to photosystem II. The phycobilisomes of red algae are particularly rich in phycoerythrin, which strongly absorbs green light and so appears red in color. However, not all red algae look red, but are often dark green, gray green, or black, because of their ability to efficiently absorb most colors of light.
Most red algae are adapted to conditions of low carbon dioxide
concentrations relative to available light energy, via a process
called biogenic decalcification. The relatively abundant light
energy is used to pump protons across membranes, which convert
bicarbonate in the water just outside the cell to carbon dioxide.
In effect, the alga creates a thin film of acidic water around its
body, from which carbon dioxide is efficiently gathered by the
alga for photosynthesis. The pH of the water further from the cell
is raised by biogenic decalcification, often to the point where
calcium carbonate precipitates on the surface of the organism.
Some algae use this calcium carbonate to strengthen their cell
walls.
Many aquarium plants lack the proton pumps for biogenic
decalcification, and those that have them are significantly less
effective at it than red algae. As a result, a brightly-lit
aquarium deficient in carbon dioxide gives a competitive advantage
to red algae. This points to two methods of control: Ensure that
the aquarium is not overly illuminated, particularly with
yellow-green light that green plants use poorly; and ensure that
carbon dioxide levels are adequate to the lighting level.
Staghorn algae, Compsopogon caeruleus, is a common
inhabitant of aquariums. It typically looks like a mass of wiry
hairs growing from hardware, rocks, or plants. Its color can vary
all the way from very light gray to black. It has a tendency to
branch in a way that gives it its common nickname, but is
quite variable in form depending on the environment in which it
finds itself. It attaches quite tightly to its substrate and so is
very difficult to remove mechanically, and there are few algae
eaters that are much interested in it. Siamese algae eaters may
pick at it but will tend to choose other foods if available.
Staghorn algae is distinguished from black brush algae by its
coarser bristles and greater tendency to branch, but the two can
be difficult to tell apart with the naked eye when still young.
Under the microscope, the two are easily distinguished, as
discussed below.
Staghorn algae is vulnerable to the glutaraldehyde in "liquid
carbon" products, and the algicidal properties of glutaraldehyde
probably account for more of the benefits sometimes seen from
"liquid carbon" than any carbon enrichment. Particularly
persistent patches of staghorn algae can be treated by squirting
part of the glutaraldehyde dose directly on the patch with a
syringe. Hydrogen peroxide can also be used to treat isolated
patches, and it leaves no residue in the tank. (This can be a good
or a bad thing, depending on whether you want residual
glutaraldehyde to continue working on patches you miss or cannot
directly treat.) Staghorn algae killed by an algicide turns
visibly red, which is useful for being sure you have correctly
identified your infestation as a red alga, and is also useful for
knowing which patches need further treatment.
This is among the more unwelcome pests in an aquarium. Black
brush algae, also sometimes called beard algae, is typically Audouinella
or Rhodochorton. It takes the form of many thin
filaments emerging from a single holdfast, which adheres tightly
to rocks, hardware, and especially plants. This gives it the
appearance of dense tufts and distinguishes it from staghorn
algae, which has thicker branched filaments. The two are easily
distinguished under a microscope, as black brush algae filaments
are always one cell wide and the cells are elongated, with
ribbon-like chloroplasts. Older staghorn algae filaments are
several cells across and the cells are more rounded. As the
common name suggests, black brush algae is dark green to
nearly black in color, though Rhodochorton can have a
reddish cast. Black brush algae can overrun a tank and it tends to
grow over green plants and smother them.
In general, black brush algae thrive under the same conditions as
staghorn algae. They strengthen their cell walls with calcium
carbonate when engaging in biogenic decalcification, making then
especially unattractive to algae eaters. It is claimed that growth
of black brush algae is favored by a high level of dissolved
organic carbon in aquarium water, so that keeping the tank clean
with frequent water changes can help combat black brush algae.
There is also some evidence that ammonium spikes can cause a black
brush algae bloom.
Like staghorn algae, black brush algae is vulnerable to the
glutaraldehyde. Siamese algae eaters are among the few algae
eaters that will nibble on black brush algae, though they are
unlikely to bring it under control on their own.
These resemble black brush algae, but are green or yellow-green
algae and so are dark to bright green in color. They adhere
tightly to surfaces (driftwood seems particularly favored) and
have a texture almost like steel wool. A number of not necessarily
closely rated species of algae fit this description, and precise
identification can be difficult even with a microscope.
These algae are almost impossible to completely eradicate from a planted tank, and many aquarium keepers choose to make their peace with them. A modest amount of green hair algae on a piece of driftwood can in fact be fairly attractive. They like almost precisely the same conditions as green plants, including lighting, and they seem impervious to glutaraldehyde. Siamese algae eaters will sometimes pick at them but they are not a preferred snack. They do seem to endure low nutrient levels better than green plants, so keeping the aquarium well fertilized for the light level, along with removing as much as possible by hand, is the best hope for control.
 |
 |
Cladophora is a chlorophyte, or green alga. The
chlorophytes are one of the two main branches of the
Viridiplantae, the other being green plants and their closest kin.
The Viridiplantae in turn are one of the two main branches of the
archaeplastids, the other being the red algae. The Viridiplantae
are distinguished by the fact that their chloroplasts have lost
their phycobilisomes and, with them, the ability to use
yellow-green light efficiently for photosynthesis.
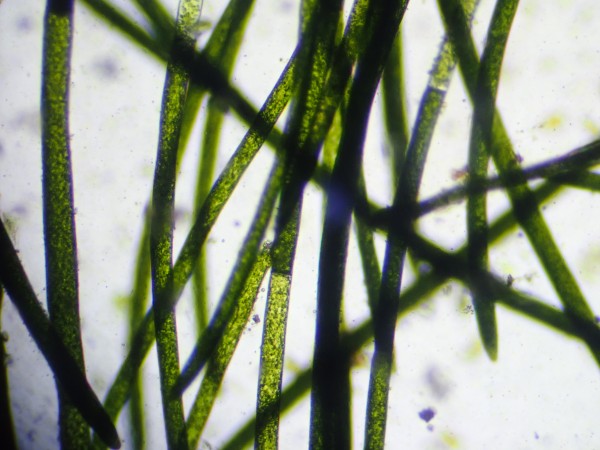
Cladophora typically forms dense tufts, often deeply
branched, that are deep green in color and are very firmly
attached to hardscape or driftwood. It it sometimes known in the
aquarium hobby as blanket weed. Its branching patterns and general
growth are variable enough that it is not easy to distinguish from
other filamentary algae even with a microscope. However, its long
cells and vivid green color distinguish it from Audoinella,
its branches and long cells lacking ring scars distinguish it from
Oedogonium, and the presence of cross walls dividing its
cells distinguish it from the siphonous Vaucheria. It
gives off a pungent smell when crushed.
Cladophora is sufficiently closely related to green plants that it is very difficult to exterminate it from a planted aquarium once established. Many aquarists regard it as the most difficult of algae pests. It likes almost exactly the same conditions as aquarium plants and it is resistant to glutaraldehyde. Siamese algae eaters will pick at it, but without much enthusiasm. Generally good tank conditions will slow its spread but will not exterminate it. The aquarium keeper either has to complete tear down and disinfect his tank, or make his peace with the alga. Fortunately, Cladophora can be fairly handsome as a growth on driftwood, which seems to be among its favorite anchoring sites. Java moss seems able to shade it out.
Rhizoclonium is a close relative of Cladophora, but it rarely branches and does not attach tightly to surfaces, forming a threadlike mass that gives it its common name of green thread algae. Under the microscope, it is not dissimilar, but has a single netlike chloroplast rather than the numerous small chloroplasts close to the cell wall that characterize Cladophora. It is also a lighter green color.
Like other green algae, it thrives under the same conditions as green plants, but is particularly common in new aquariums. It is easily removed mechanically and is relished by most algae eaters and so rarely becomes a serious problem.
Oedogonium is another green algae, somewhat more distantly related to Cladophora. It forms unbranched filaments that cling tightly to surfaces, giving it its common name of fuzz algae or hair algae. It can be distinguished under the microscope from the presence of rings or scars near the ends of its cells.
Though difficult to remove manually, it is attractive to many algae eaters, which can usually bring it under control. Shrimp are particularly fond of it.
Spirogyra is among the closest relatives of green plants
that is not considered a green plant. It forms very long threads,
not usually attached firmly, that feel silky or oily to the touch
because they have a coating of mucilage. This gives it its common
name of water silk. Under the microscope, it shows distinctive
spiral-shaped chloroplasts from which it gets its name.
It can be removed manually. Unfortunately, few algae eaters relish it.
Vaucheria can be difficult to distinguish from Cladophora,
even with a microscope, in spite of the fact that the species are
not closely related. Vaucheria is a xanthopyte, or
yellow-green algae, and is a close relative of kelp and other
marine brown algae and a more distant relative of diatoms. Like
diatoms, it is an ochrophyte, and its chloroplasts lack
phycobilisomes but contain an unusual carotenoid called
diadinoxanthin. Otherwise it uses approximately the same
wavelengths of light as green algae and green plants.
This is is a less common interloper in aquariums, and, in the
wild, it tends to grow right at the waterline. Not much is known
about how it is best controlled.
Coelochaete on aquarium glass |
Microscopic view of Coelochaete on cryptocoryne leaf. The coelochaete is the green patch crossing the leaf diagonally. Note the green chloroplasts in the leaf cells, which strongy resemble the algae cells. |
Green spot algae are typically coelechaetes, colonial green algae
that are very close relatives of green plants. Like many other
algae, they are almost always present in a healthy aquarium and
can never be entirely eradicated. They form green spots on just
about every surface in the tank, which adhere very tightly.
The green plants are thought to have evolved from a coelochaete,
since coelochaetes closely resemble liverworts, the most primitive
of land plants. The connection is supported by genetic sequencing.
It is therefore no surprise that their chloroplasts are nearly
identical. This also means that green spot algae likes nearly the
same lighting and other tank conditions as many aquarium plants.
They are thought to have a competitive advantage over green plants
if the photoperiod is too long (more than 8-10 hours) or the light
too iintense for the available nutrients (especially if phosphate
is low).
Those that colonize the glass can be removed with an algae scraper, but those that colonize plant leaves are all but impossible to removed. The best control, other than overall balanced tank conditions, are algae eaters such as snails, otocinclus, ancistrus, or Siamese algae eaters. All enjoy young colonies of green spot algae, though they are unlikely to keep the aquarium walls completely clean or prevent a slow buildup of green film on the slowest growing plants. Otocinclus are particularly useful for removing some green algae from plant leaves, while nerite snails and ancistrus are a good alternative to larger plecostomus for keeping glass and other hard surfaces cleaner.
This is a very generic term for any single-celled or semicolonial motile alga that likes to settle on hard surfaces in an aquarium, particularly the glass. It turns out that "single-celled motile alga" covers many widely separated branches of the tree of life. Green dust algae do not adhere tightly to the surfaces, which distinguishes them from green spot algae. However, when scraped from the surface, the individual algal cells simply settle back on the surface after a few minutes.
Most infestations are self-limiting. They seem to occur in conditions of ample light and nutrients as an algal bloom, which is eventually brought back under control by various microscopic and macroscopic predators. Of the latter, ancistrus seem particularly fond of greed dust algae.
This is a general term for any free-swimming alga that blooms in
the tank water and turns the water itself opaque green. A great
variety of organisms scattered across the tree of life can present
themselves this way. In nature, these are all part of the phytoplankton,
the photosynthesizing organisms at the base of the food chain,
which are eaten by zooplankton such as Daphnia or Cyclops
that in turn are eaten by small fish. In our aquariums, fish
quickly consume zooplankton and we must supplement with fresh,
frozen, or prepared fish foods.
Causes and control are similar to those for green dust algae.
More persistent infestations may be brought under control by
filtering with a diatom filter, or by circulating the water
temporarily through an ultraviolet water purification unit.
Long-term control usually means maintaining generally healthy tank
conditions and letting a balanced community of microorganisms,
including predatory microorganisms, develop in the tank.
Chlorella are single-celled chlorophyte algae closely related to cladophora. Under the microscope, the cells are spherical and lack flagella. Because of its potential for rapid reproduction using simple nutrients, and its high photosynthetic efficiency (it can capture up to 8% of the light energy directed at a culture of the alga) it has been considered both as a food source and as an atmosphere regenerator for spacecraft on long missions. Ankistgrodesmus and Scenedesmus are distantly related chlorophytes consisting of spindle-shaped or ribbon-shaped cells, but with similar characteristics.
Because none of these algae are motile, they are relatively easy
prey for small predators. Water fleas, Daphnia, are
particularly fond of them; unfortunately, fish are particularly
fond of Daphnia. One solution is to place the Daphnia
in a large breeder net, designed to protect tiny fry from larger
fish. Smaller organisms such as ciliates can also feast on them
and will eventually bring a bloom under control, sometimes very
abruptly. As a result, a chlorophyte green water bloom often needs
no treatment other than watchful waiting.
Water changes may actually be counterproductive, since the predators breed more slowly than the green algae. You will remove both predators and prey, and the prey multiply to fill the void more rapidly.
Flocculants are chemicals which help bind small particles together. Flocculants sold for aquarium and pool use can clump Chorella and its relatives, making them easier to filter and also easier prey for predators. Be warned that some flocculants use metal salts that may be hard on plants and fish; others use relatively benign polymers. When an aquarium is treated with a flocculant, the mechanical filter can plug up very quickly, so it should be closely monitored.
Euglena are very odd critters indeed. They are excavates,
possibly the oldest line of eukaryotes, which consists of
unicellular organisms with varying numbers of flagella. The
euglenoids are the only excavates that conduct photosynthesis, and
their ancestori is thought to have acquired its chloroplast by
assimilating a single-celled green alga. Thus their photosynthesis
is very similar to that of green algae and green plants.
However, most euglenoids retain a pair of flagella and a
cytostome ("cell mouth") allowing them to prey on smaller
organisms for additional nutrients. This makes them less easy
targets for other predators, so that a green water infestation of
Euglena is likely to be more persistent than other kinds.
Because the organisms are motile, they will move around to where
the light is best, so if one end of the aquarium is darkened and
the other lit, they will move to the lit end. Flocculants
are also less likely to be helpful.
Persistent infestations may require blacking out the aquarium, as
for cyanobacteria, or temporary use of an ultraviolet water
purification unit. As a last resort, treatment with copper is effective.
These are cryptomonads, single-celled algae with two flagella and
one or two chloroplasts. The chloroplasts are apparently engulfed
red algae cells; they have four membranes, and there is even a
vestigial nucleus within the first pair of membranes. Like
euglena,they are motile and poor targets for predators, and
treatment is likely to be similar.
The usual algae eaters rarely consume much black brush or green hair algae. Even Siamese algae eaters are unlikely to keep heavy growths of these forms under control. This does not mean that an aquarium should not have some algae eaters. I consider Malaysian trumpet snails and otocinclus as near mandatory and larger snails (mystery or nerite) and an ancistrus as highly desirable. Malaysian trumpet snails help keep the substrate aerated and clean up debris,while otocinclus are unexcelled for removing softer algae from plant leaves.
It should not be unexpected that an aquarium will eventually acquire a population of algae that are unappetizing to whatever algae eaters have been placed in the tank. This is a natural consequence of the selective pressure applied by the algae eaters. They are eradicating those algae types that are not visible in the tank.
Although Siamese algae eaters are among the best for consuming the tougher forms of algae, I have grown a bit skeptical of them. They much prefer whatever else is available to eat in the tank and will feed heavily on the tougher algae only if kept nearly starved, which is impractical in a tank containing any non-algae eaters. They also grow rather larger than most novice aquarium keepers realize, often to 6" or better, but their appetites do not grow in step. Instead, they dominate competition for flake food or whatever other food supply is being offered the other fish.
Lately some species of gara, such as
Garra gotyla gotyla, have become available on the market
and are claimed to be excellent black brush algae eaters that do
not grow larger than 3" or so. Information is still lacking,
but some sites describe these as omnivores, which makes me
skeptical. I also have no information on whether they are inclined
to eat green plants as well as algae. To coin a phrase: I will
follow their career with great interest.
It is also important to properly subsidize algae eaters of all types. A population of otocinclus that is large enough to scrub an aquarium clean of softer algae is a population of otocinclus that is in danger of starvation if not offered supplemental food. This can take the form of algae wafers or of blanched vegetables (such as peas, leafy greens, or sweet potato) periodically placed in the tank as a treat. These should not be supplied to excess so as to spoil the appetites of the algae eaters, but should be sufficient to ensure they have enough to eat. An oto with a round fat belly is a happy oto; if the otos have round bellies but are continuing to scrub surfaces in the tank, then you have struck the right balance.
Some species of snails are added deliberately to planted
aquariums and seem to be good citizens. Among the best are nerite
snails, which have a good reputation for leaving live plants
alone.

They sometimes make themselves a nuisance by laying large numbers of small white eggs on driftwood, rocks, or the aquarium walls, but these require brackish water to develop and so will not hatch in a freshwater aquarium. Because nerite snails cannot reproduce in fresh water, they almost never appear uninvited. This is not true of several other common species of snails.
Malaysian trumpet snails are often regarded as beneficial,
since they burrow through the substrate looking for decaying
organic matter to feed on and help loosen and aerate the
substrate.
They rarely if ever feed on live plants. However, they are parthenogenic,
meaning they are capable of producing fertile eggs without a mate;
and they are oviviparous, meaning they carry their eggs in
a brood pouch in which the eggs hatch. The eggs do not receive
nourishment from the mother snail, only protection. The snails can
begin reproducing while quite young and can produce up to 70
offspring per brood. This mode of reproduction means that a single
Malaysian trumpet snail with an ample food supply can become
hundreds with astonishing speed. They are the tribbles of the
aquarium! While they are often deliberately introduced into the
aquarium, they can easily come in uninvited as young snails
clinging to plants.
Wild snails that come in with live plants are also a risk for plant damage. These are often described generically as "pond snails", but true pond snails (Lymnaea) are only one genus of wild snails that may find their way into an aquarium uninvited. Like Malaysian trumpet snails, many are parthenogenic and can quickly multiiply; those that are not parthenogenic are often hermaphroditic, so that any two can interbreed. Unlike Malaysian trumpet snails, they will often feast on aquarium plants, particularly when other food becomes inadequate.
The first and surest way to avoid introducing unwanted species of snails into an aquarium is to plant your aquarium only with plants raised in tissue culture. The plants are raised in a near-sterile environment on nutrient gel from small bits of plant tissue, and are guaranteed to be free of snails or parasites. This technique was adopted decades ago by orchid growers, but it is now becoming popular for aquatic plants, and several varieties are available at local fish shops. The chief drawbacks are that the plants are somewhat expensive, they sometimes take a long time to become established in the aquarium, and the choice of species is still limited. Species I have seen for sale include hygrophyla, cryptocorynes, bacopa, anubis, echinodorus, Java fern, staurogyne, and alternanthera. Species I have not been able to find in tissue culture include vallisneria, stem plants other than bacopa or alternanthera, or Java moss.
The second way to prevent snail infestation is to disinfect plants that are not tissue
cultured.
The first question to ask yourself is whether you want to truly eradicate snails, or are content with merely controlling their numbers.
If you are willing to tolerate a few snails in your tank, then
the first step is to be scrupulous about not overfeeding your
fish. Snails eat whatever your fish don't, and their
multiplication rate depends on how much food they have. Your fish
should have consumed all the visible food within five minutes or
so of feeding, and should be scouting the tank for anything they
missed. The old entertainer's rule applies: Leave them wanting
more. If you inadvertently overfeed your fish, you may wish to
make a small water change in which you take the opportunity to
vacuum up any remaining food particles. This is often the only
control needed for Malaysian trumpet snails.
The second step is to cull snails. This means periodically picking every snail out of the tank you can find and disposing of it. The process can be made easier by leaving out something the snails like, such as a lettuce leaf or slice of cucumber, so that the snails congregate around it and are more easily culled. If you are squeamish, you can put the bait on a plate or in a small dish and simply pick up the entire plate or dish when it is filled with snails. There are also snail traps available online that have a place for bait (sometimes sold with the trap) and a mechanism to make it more difficult for the snails to leave once they have tasted the bait. I have not found snail traps particularly effective (except at trapping particularly dimwitted small fish) and do my culling by hand.
There are some fish that are fond of escargots and can substantially cut back your snail population. These include clown loaches, which are delightful fish when young and have a big appetite for snails. Unfortunately, they become quite large, are less voracious of snails once they are big enough to snarf down more than their share of fish flakes, and they sometimes develop a taste for prized aquarium plants. Zebra loaches and dwarf chain loaches are better choices. Other snail-eating fish included bettas and gouramis. Bettas are normally kept singly, but a single male betta or a small number of female bettas can be included in a community tank.
A rather surprising but highly effective predator for snails is
the assassin snail, Anentome helena. This is a carnivorous
snail that happily feasts on other snails, including ones larger
than itself. As a predator, its reproduction rate is much less
than other snails, but it is understandable if replacing one snail
species with another is not entirely appealing. This species can
reproduce in fresh water, but is not parthenogenic or
hermaphroditic, so that a male and female must be in the aquarium
for them to breed.
If you wish to genuinely eradicate all your snails, you will have
to turn to molluskicides, such as copper.
Keep in mind that molluskicides are invariable hard on fish and
plants, not to mention desirable invertebrates.
These include everything from harmless organisms that generally
go unnoticed to organisms who are essential for the health of an
aquarium.
These are bacteria that oxidize ammonium to nitrite or nitrite to nitrate or both. As such, they are absolutely essential in an aquarium, and they are "uninvited" only in the sense that we may not have specifically introduced them into the aquarium, relying instead on their presence in small numbers almost everywhere to provide the initial culture.
These Gram-negative motile rods are members of the
betaproteobacteria. Nitrosomonas oxidize ammonium to
nitrite. They dislike light and so will tend to form biofilms or
clumps in shaded locations where there is good water flow and
abundant oxygen. For this reason, biological filters should be
designed to exclude light. Nitrosomonas is capable
of using energy derived from ammonium oxidation to fix atmospheric
carbon dioxide, and some varieties are able to decompose urea,
CO(NH2)2, into carbon dioxide and ammonia.
This is a genus of Gram-negative aerobic spiral bacteria that went unnoticed even by scientists until 1985. They are now known to be present in almost all oxygen-rich environments, including specifically the biological filters of mature aquariums. They are capable of oxidizing nitrite to nitrate and there is now evidence they can oxidize ammonium to nitrate in a single process. This makes them among the most welcome of aquarium guests, and there are cultures containing Nitrospira for inoculating aquariums on the market. Nitrospira tends to aggregate into clumps within the biofilm on aquarium surfaces wherever there is flowing water to supply ammonium, nitrite, and other nutrients, and oxygen for ammonium and nitrite oxidation.
This is a genus of nonmotile gram-negative rods that are members
of the alphaproteobacteria, cousins of Nitrospira and Nitrosomonas.
They carry out the second step in the nitrogen cycle, oxidizing
nitrite to nitrate.They are slow-growing and have a distinctive
yellow color that contributes to the orange-brown color of the
biofilm that forms on biological filters.
This is a genus of gammaproteobacteria, relatives of Nitrosomonas.
Like Nitrobacter, they carry out the second step in the
nitrogen cycle, oxidizing nitrite to nitrate.
These are bacteria that live within the digestive tracts of our regular tank inhabitants, in a mutualistic relationship that benefits both.
Bacteroides are Gram-negative anaerobic rods found in the digestive tracts of many animals. There is now pretty good evidence that some freshwater fish get their supply of Vitamin B12 from Bacteroides in their digestive tracts. You do not need to supply these bacteria; they come with the fish. Feed the fish a healthy diet, and their gut bacteria will feed them the Vitamin B12 they need. Those fish that do not have gut bacteria that produce Vitamin B12 must obtain it in their diet. In nature, this comes up the food chain from phytoplankton, such as Chlorella, that get their B12 from symbiotic bacteria that scientists are still working to identify.
The majority of bacteria in an aquarium are incapable of photosynthesis (phototropism) or of extracting energy from inorganic chemical reactions (chemotropism) and so must feed on organic molecules already present in the aquarium (heterotrophism). Those that feed on living organisms, including our regular aquarium inhabitants, are actually pretty uncommon. Most feed on dead organic matter, decomposing it into dissolved organic carbon, or feast on dissolved organic carbon, decomposing it into innocuous carbon dioxide.
It is surprisingly difficult to pin down which bacteria are most important in natural bodies of water. Culturing samples turns out to be misleading, because a number of important bacterium species simply don't grow well on culture media, and some do not grow well in pure culture; they are symbiotic with other organisms. However, modern methods of molecular biology overcome some of these difficulties. Water samples can be analyzed for miniscule quantities of RNA from the bacteria living in them, the RNA sequenced, and the sequences compared against RNA libraries to get a pretty good census of which bacteria are most numerous.
One surprise is that actinobacteria are among the most numerous bacteria in freshwater lakes, sometimes making up over half the bacteria in the surface layers. Actinobacteria are mostly Gram-positive aerobic rods with a tendency for daughter cells to remain joined after cell division. This produces long strings of cells that were long mistaken by biologists for a primitive form of fungus. Their biochemical capabilities are diverse and impressive. Some generate antibiotics while others are among the few organisms that can break down cellulose. However, those present in freshwater do not culture well and are often dependent on other bacteria, so their importance long went unrecognized. The species that favor freshwater environments are unusually small for this phylum. Some appear to have a form of bacteriorhodopsin, a photosynthetic pigment that may allow them to extract a limited amount of energy from green light.
The proteobacteria are overwhelmingly beneficial decomposers, of
which only a handful occasionally turn nasty. The most common in
lakes are betaproteobacteria, followed by alphaproteobacteria and
gammaproteobacteria.
Alphaproteobacteria are capable of growing at very low nutrient levels and are thought to be the ancestors of the mitochondria in eukaryotic cells.
Betaproteobacteria have amazingly diverse biochemical capability, and include ammonium- and nitrate-oxidizing bacteria.
Gammaproteobacteria are a very large group that include the pseudomonads.
This is the phylum from which Bacteroides and columnaris
comes. It is the third most common phylum found in lake
environments. They are Gram-negative and mostly rods, and those
that live in open water rather than in fish guts are aerobes,
facultative anaerobes, or aerotolerant. They tend to cling to
particles in the water and are particularly good at breaking down
large organic molecules.
This is a recently recognized phylum of bacteria that turns out to be quite common in natural waters. It gets its name because of the warty appearance of the bacteria, which have all sorts of knots and protuberances on their surfaces. An interesting feature of this group is that they have genes for tubulins, proteins previously thought to be restricted to eukaryotic cells. Tubulins form the "skeleton" of eukaryotic cells. It has been speculated that the original eukaryote was a mutated verrucomicrobium.
Archaea are organisms which superficially resemble bacteria. They
are organisms of a single cell or small group of cells and the
cells are a single compartment. However, their cell membranes have
subtly different chemistry from those of bacteria or eukaryotes.
Their DNA resembles bacterial DNA but is transcribed to proteins
in a way that more closely resembles eukaryotes. It is thought
that the first eukaryote was an archaeon that engulfed an
alpha-proteobacterium, so there is a sense in which we are
descended from both.
We are only beginning to understand this large and diverse group. The original examples all lived in extreme environments, but we now know that they are present, and even common, in ordinary environments such as natural bodies of water. They are almost certainly present in a planted aquarium. There are no known archaean parasites, so they seem to be remarkably good citizens. However, we know almost nothing beyond that of what roles they play in a planted aquarium.
Many of the methods used to control pest or parasites are fairly nonspecific. Rather than repeat their description for each organism they are effective against, we list them once here for reference.
These are control methods aimed at preventing an infestation from taking hold in the first place.
Disinfection is the use of chemical or physical agents to reduce
the numbers of microorganisms on aquarium equipment or hardscape.
Disinfection is mostly a means for preventing pests from spreading
between aquariums.
Most disinfectants safe for household use are not
sterilants; that is, they do not destroy all microbial life. This
is normally not necessary for the aquarium keeper. Biologists
group microorganisms, from most resistant to disinfection to
least, as endospores > mycobacteria > nonenveloped viruses
> fungi > vegetative bacteria > enveloped viruses.
Endospores, which are a resting stage of some bacteria, are very
difficult to kill but are also of little concern in the aquarium.
The only mycobacterium of concern is the mycobacterium that causes
fish tuberculosis, and this is uncommon. The only virus of
any concern to most aquarium keepers is the dwarf gourami
iridovirus, which is an enveloped virus and very easy to kill.
Fungi are rarely of concern. Vegetative bacteria are bacteria that
are not endospores. Not appearing in this ranking are two groups
of great concern to aquarists: tomonts of parasitic protozoa,
particularly Ichthyophthirius, and spores of algae pests.
My best guess is that algal spores are similar in resistance to
fungus (which is also often spread by spores.) Since ich is
probably the single greatest worry for transmission from aquarium
to aquarium, and is likely more resistant than vegetative bacteria
or even algal spores due to the thick mucus coat of the tomonts,
chemical disinfectants for aquariums are best evaluated on the
basis of their ability to destroy ich tomonts and fungus.
Unfortunately, good information on how resistant ich tomonts are
to a particular disinfectant are often lacking.
Regardless of the method of disinfection used, disinfection is
likely to be more effective if the item being disinfected is
thoroughly washed first, preferably in a mild detergent. Live
plants may be able to tolerate a brief wash in mild detergent
solution but should not be left in long and should be thoroughly
rinsed afterwards.
The simplest rapid form of disinfection is heating to a
temperature sufficient to kill the unwelcome organisms. The required
temperature depends on the organism. For example, laboratory
tests have shown that tomonts of Cryptocaryon, a marine
parasite similar to ich, are vulnerable to 1 hour exposure to
temperatures of 40°C (104°F). At the other extreme,
bacterial endospores are resistant to prolonged boiling and can
be reliably destroyed only in an autoclave (a laboratory
pressure cooker) or by pyrolysis, direct exposure to a
burner flame. However, the kinds of bacteria that we worry
about most in aquariums cannot long tolerate a temperature of
160°F (71°C), so even a minute of boiling (212°F or 100°C)
should reliably disinfect any aquarium pests.
Obviously, we
cannot disinfect live plants by boiling them. Heat disinfection
is thus useful only for aquarium equipment or hardscape. Large,
porous objects, such as driftwood, must be boiled long enough
for the heat to penetrate completely into the object.
Some less resistant parasites and pests can be destroyed simply by drying equipment thoroughly. For example, ich tomonts have little resistance to dessication and are destroyed when equipment or hardscape they have attached to is dried for 24 hours. However, dessication will not reliably destroy many vegetative bacteria, protozoa, algal spores, and viruses.
Household bleach is a solution of 5% to 8% sodium hypochlorite, NaOCl. This is a source of hypochlorous acid, HOCl, which is the actual killing agent, via the reaction
OCl- + H+ -> HOCl
with a pKa of 7.53. Thus bleach is more effective at neutral to acid pH. However, acidifying bleach solutions is dangerous, because of the further reaction
HOCl + H+ + Cl- -> Cl2 + H2O
with equilibrium
[HOCl][H+][Cl−]/[Cl2] = 3.9x10-4 M2
which produces poisonous chlorine gas at low pH. This reaction
requires chloride, but this is always present in bleach solutions,
because bleach is typically prepared by reacting chlorine gas with
a sodium hydroxide solution. This is effectively the reverse of
the last reaction.
Because both chlorine and hypochlorous acid are effective
disinfectants, and because of the rapid equilibrium between them
and with hypochlorite (OCl-), the disinfectant power of
bleach solutions is expressed in terms of free chlorine, which is
defined as the amount of chlorine present as dissolved chlorine,
hypochlorous acid, and hypochlorite. Bleach is prepared as a
highly alkaline solution, with a pH around 12, because this shifts
the equilibrium towards the highly soluble hypochlorite ion and
away from the volatile and toxic chlorine. This makes the bleach
safer to handle while simultaneously increasing the amount of
chlorine that can be dissolved in the bleach.
Free chlorine makes up a little under 50% of sodium hypochlorite by weight. Thus, one ml of 5% bleach per gallon of water corresponds to about 6 mg/l free chlorine. Destruction of Cryptocaryon tomonts requires exposure to ten times this concentration, 60 mg/l free chlorine, for 24 hours. Increased concentration and higher temperatures reduce the required time of exposure; for example, disinfection of equipment or hardscape requires an exposure time of an hour at a concentration of 100 mg/l of free chlorine at a temperature of 50C (120F). Fifteen minutes is sufficient for thorough disinfection with 10% bleach, equivalent to 4000 mg/l free chlorine. Concentrations greater than 10% are counterproductive, since the solution will be so alkaline that little hypochlorous acid will be present. At the higher concentrations, bacteria are destroyed within seconds, and with longer soaking, chlorine will destroy many endospores, though it is not rated as a true sterilant.
Bleach is inexpensive to use and can be highly effective. Its chief drawbacks are that it is slightly corrosive, it smells, and it is easily neutralized by reactions with organic matter. Anything disinfected with bleach should first be thoroughly washed to remove extraneous organic matter (this is good advice for all disinfectants). Disinfected objects should be rinsed with water containing dechlorinator before being moved into the aquarium.
Keep in mind that household bleach tends to contain other ingredients and to vary in concentration. It should be plain bleach, without citrus scents or other additives. Bleach sold as "concentrated" or "extra strength" may have up to 8% sodium hypochlorite; check the label, and remember that inert ingredients may not all be listed. The aquarium keeper may be better off buying calcium hypochlorite sold for use in pools, which also has dosing information for achieving the desired concentration. Pure calcium hypochlorite, Ca(ClO)2, is a fairly stable powder that provides about 50% free chlorine by weight.
Bleach soaks are sometimes recommended for disinfecting live plants. For example, several Internet sites recommend disinfecting plants in a 5% bleach solution (1250 mg/l free chlorine) for two minutes. This seems sufficient to destroy almost all vegetative bacteria and possibly algal spores and ich tomonts, if your plants can withstand this treatment. If you try this, the plants should be rinsed in water dosed with a dechlorinator before being moved into the display tank.
Isopropanol (isopropyl alcohol) and ethanol (ethyl alcohol) are
both excellent disinfectants; in fact, the Centers for Disease
Control regards them as underrated. They can rapidly destroy all
vegetative bacteria, mycobacteria, fungus, amoeba cysts, and all
enveloped viruses and many nonenveloped viruses. Their
effectiveness against amoeba cysts suggest they can be effective
against ich tomonts, but this is not guaranteed (since tomonts
have a thick mucus layer) and I have found no reports of
experimental results. Isopropanol is slightly more effective than
ethanol, but both must be used at concentrations between 70% and
90% for best effectiveness. Diluted alcohol is all but useless.
Oddly, pure alcohol is also ineffective. Apparently some water
must be present for the alcohol to denature proteins. Disinfection
is rapid, taking place in seconds for vegetative bacteria and in
twenty minutes for the most resistant fungus spores.
The chief drawbacks of alcohol are that it does not affect
bacterial spores (which we need not usually worry about), must be
used at full strength (making it somewhat expensive to use), and
is volatile and flammable. Also, it seems very unlikely that live
plants can survive immersion in 70% alcohol for even a few
seconds. Because alcohol rapidly evaporates, it is not enough to
wipe it on a piece of equipment; the equipment must be immersed in
the alcohol. Alcohol may dissolve some plastics and coatings and
should be tested first on an inconspicuous location.

Benzalkonium
chloride is a quaternary ammonium compound, which is
conceptualized as an ammonium molecule where each of the
hydrogen atoms has been replaced by a hydrocarbon group.
Quaternary ammonium compounds kill vegetative bacteria,
protozoa, and enveloped viruses by breaking up the lipid bilayer
making up the cell membrane or viral envelope. They are
ineffective against bacterial endospores, mycobacteria, protozoa
cysts, some Gram negative vegetative bacteria (such as Columnaris),
and nonenveloped viruses.
Benzalkonium chloride is readily available on the Internet and is mild enough to be used in consumer products, including as a preservative in eye drops, but it can be dangerous in higher concentrations. The maximum concentration in eye drops is 100 mg/l. This has been shown to be effective at killing Cryptocaryon tomonts after an hour's exposure. It seems worth testing whether aquatic plants can tolerate it at 100 mg/l for this long. The procedure would be to make up the proper concentration of benzalkonium chloride in water, immerse the plants for a full hour, and then rinse thoroughly before moving the plants into the aquarium.
Both alcohol-formaldehyde and hospital-grade glutaraldehyde are true sterilants. They are overkill for any reasonable aquarium use and are expensive and dangerous to use. However, both formaldehyde and glutaraldehyde have been used for control of pests in aquariums, and we will revisit them again shortly.
Hydrogen peroxide is a reasonably effective disinfectant in dilutions of up to 0.5%, though it may take as long as an hour to have its full effect. It is sold in drug stores as a 3% solution, so this would correspond to about a 1:6 dilution in water. Higher concentrations sold in hospitals are capable of destroying endospores and so are true sterilants, but this is overkill and less expensive disinfectants are a better choice.
As with bleach, hydrogen peroxide is sometimes recommended as a
disinfecting soak for plants. Whether any plants can actually
withstand an hour soak at 0.5%, the minimum effective
concentration, is unclear. I know from experience that Vallisneria
cannot. I have seen Internet sites recommend a soak in a
solution of 3 ml of 3% peroxide per gallon of water for five
minutes, but it is most unlikely that this dilute a solution will
accomplish anything.
This obsolete disinfectant is still sometimes recommended in the
aquarium trade. It was once used to disinfect water supplies, but
was replaced with hypochlorite, itself now replaced with chlorine
gas. Permanganate now seems to be used in water treatment mainly
as a pretreatment to oxidize iron and manganese before the water
is disinfected with chlorine. Permanganate is unsuitable for
equipment or hardscape because it is staining, and I am deeply
skeptical it can be effective as a plant disinfectant at the
concentrations and exposure times sometimes recommended at
Internet sites.
For disinfecting an entire aquarium that has been torn down and
is going to be rebuilt, I think you can do no better than to use a
10% dilution of household bleach in water and completely fill the
tank. For a 200 liter tank, this requires 20 liters of bleach, but
bleach is inexpensive and effective. The tank should soak for a
full hour to ensure that the bleach penetrates everywhere. If you
want to be stingy on the bleach, you can try a lower concentration
for a longer time at a higher temperature; use hot water dosed
with 4 ml of 5% bleach per liter (800 ml in a 200 liter tank) and
let it soak for a full day. However, the lesser amount of bleach
is more susceptible to neutralization by residual organic matter
in the tank.
For disinfecting nonporous equipment and hardscape, 10% bleach is
also the best choice. Fifteen minutes should be sufficient time.
For small items such as nets, immersion in 70% to 90% alcohol for
a full minute is another option.
For porous hardscape, such as driftwood, the best choice is boiling if the hardscape can withstand it. Thick driftwood may have to be boiled for up to an hour to ensure it is heated through. This also has the advantage of ensuring that the driftwood is thoroughly waterlogged and leached of any substances that might encourage a bacterial bloom.
In spite of the many recommendations you will find online for
disinfecting live plants, there are few disinfectants that will be
effective without killing the plants. Much depends on what you are
trying to achieve. If you are merely trying to avoid introducing
ich or other parasites into your tank, quarantine is a better
option. If you are trying to avoid introducing algal spores,
benzalkonium chloride seems worth experimenting with. If you are
out to destroy snail eggs, your best bet may be a bleach soak, if
your plants can withstand it.
Quarantine, like disinfection, is employed mostly to prevent the spread of pests between aquariums. It is used for living organisms that we wish to introduce into an aquarium, such as live plants, fish, or invertebrates.
The idea behind quarantine is that parasites will reveal themselves, given a host and enough time. The critter being quarantined is placed in a tank large enough for it to be comfortable for up to four weeks of quarantine. Water conditions are made as comparable as possible to the ultimate destination aquarium, often by filling the quarantine tank with change water from the destination aquarium, and the temperature is adjusted to be around 27C (80F). The somewhat warm temperature is to speed the life cycle of any parasite present in the tank. Because the critter has already endured the stress of being moved into the quarantine tank, the lighting is usually kept subdued.
The tank should have hiding places for shy critters and may have
some substrate, but should otherwise be bare. Some aquarists keep
a few fish or snails in their quarantine tank to keep the
biological filter turning over. Others keep the filter turning
over by feeding the tank an artificial ammonium source.
Live plants and invertebrates are put in quarantine to ensure that any parasitic tomonts are starved out. This takes about a week if there are no fish in the tank. Otherwise, the quarantine period is at least two weeks and ideally a month. Quarantine must be long enough that even fish with a light starting infestation and partial resistance will have time to show telltale symptoms.
During the quarantine period, any fish are closely inspected daily for any signs of infestation. If the fish seem healthy and happy for the entire quarantine period, they can be moved into the destination aquarium. If there are any signs of illness, the clock is reset on quarantine from the time all symptoms of illness disappear. If a parasite does manifest itself, the quarantine tank can be treated more easily than the destination aquarium, since it is usually smaller and has no plants or other inhabitants that would be adversely impacted by medication.
Once a pest gets into your aquarium, there are various ways to try to eradicate the pest without killing your fish or plants. Because you have a specific target for eradication, your approach can be more carefully tailored than general disinfection or quarantine. However, many treatment methods are applicable to more than one pest.
Any activated charcoal filter must be removed from the aquarium before treatment with any medication. Many medications can damage the biological filter and so ammonium levels should be closely monitored during their use.
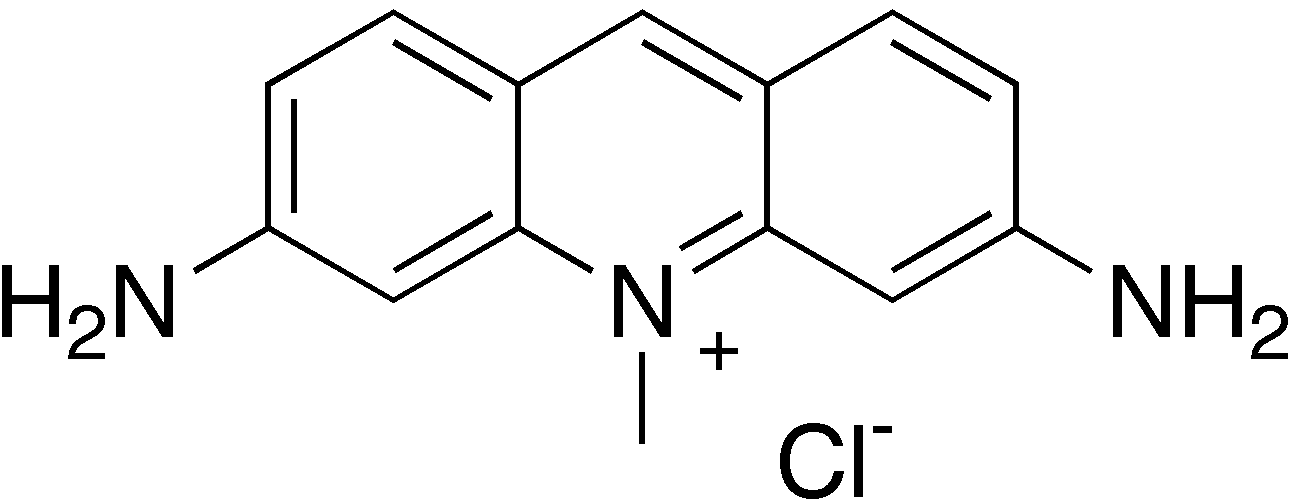
Acriflavine is a coal tar dye, with an orange-brown color. It is
effective against Oodinium, Saprolegnia, columnaris,
and other superficial bacterial infections, although the mechanism
of action is not well understood. It is less expensive than most
antibiotics and it is reputedly less dangerous for scaleless fish
than malachite green. It is also reputedly safe for biological
filters. However, it discolors aquarium equipment, and it is
lethal to live plants, so for the planted aquarium keeper, it is
strictly a hospital tank treatment. It is also lethal to
invertebrates.
Acriflavine is less effective at acid pH, and it is decomposed by
bright light. Dosing is in the ballpark of 20 mg/l.
Antibiotics are chemical substances that very strongly inhibit
very specific biological processes. Most are produced by
microorganisms or are derived from antibiotics produced by
microorganisms, which likely get a competitive advantage from
poisoning their competitors. A good example is penicillin, one of
the first antibiotics, which is produced by a fungus and targets a
specific chemical reaction used by bacteria to build their cell
walls. Penicillin is lethal to susceptible bacteria even at tiny
concentrations, but it is practically nontoxic to almost every
other form of life.
In the aquarium, antibiotics are used primarily against bacteria. Their chief drawbacks are that they often destroy beneficial bacteria as well, including those in the biological filter; they are expensive; and their overuse breeds resistant strains of bacteria, so that their use rightly tends to be highly regulated. Generally speaking, only older antibiotics that are no longer frequently used for human disease are likely to be sold in fish shops.
The dosing information below assumes that you are dosing the entire water column. If your fish are sick enough that they are not eating, as is often the case, you have little choice about this. Otherwise, you can consider mixing the antibiotic into the food supply, so that more of it gets inside the fish and less in the water column where it can destroy beneficial bacteria. Some antibiotics are sold as medicated feed, but these are aimed more at the commercial fish industry than at home aquarium keepers.
Kanamycin acts by gumming up bacterial ribosomes. All bacterial ribosomes are sufficiently alike that kanamycin is lethal to the majority of bacteria, but eukaryotic ribosomes are sufficiently different from bacterial ribosomes that a lethal concentration for bacteria is almost harmless to eukaryotes. This makes kanamycins an excellent broad-spectrum antibiotic, though its side effects have relegated it to second-line duty in human medicine. It is as lethal to ammonia oxidizing bacteria as any other and so will destroy your biological filter. It has the decided advantage that is is readily absorbed through fish skin and so is relatively effective at treating internal infections.
Dosing is 12-15 mg/l and should be repeated every 48 hours, with a preliminary partial water change.

Tetracyclines are a large family of antibiotics (first marketed
in 1950) that act by jamming the ribosomes of bacteria, in a
manner similar to kanamycin. The oldest tetracyclines have been
around long enough that many human pathogens have developed
resistance to them, and some of these tetracyclines are now
available over the counter at fish shops or on the Internet. They
are as lethal to ammonia oxidizing bacteria as any other and so
will destroy your biological filter.
The standard chemical treatment for ich is a combination of
malachite green and formaldehyde. This combination is also
used for oodinium and Saprolegnia.
Malachite green is a drug that neither contains malachite (or any
other copper compound) nor is really green (it is closer to sky
blue in color).
Formaldehyde is a very simple, rather toxic molecule famous as the preservative for specimens being dissected in biology classes.

Malachite green plus formaldehyde is highly effective at killing
ich therodonts, as well as a number of other organisms in kingdom
Harosa. Either chemical can be effective by itself, but the
combination is synergistic, meaning it is much more
effective than either medication alone. It is speculated that
formaldehyde attacks cell membranes, which allows better
penetration of malachite green to the vital structures within.
Standard dosing is 0.05 ppm malachite green and 15 ppm
formaldehyde. However, I do not recommend formulating your own
mixture; ich is not something to screw around with, and neither
are formaldehyde and malachite green, both of which are suspected
carcinogens. I generally refrain from specific product
endorsements (or criticisms), but I have gotten excellent results
with Kordon's
Rid-Ich Plus (I do not recommend their "organic"
product) against strains that were resistant to both heat
treatment and malachite green alone. Use exactly as directed,
which includes a daily 25% water change before administering the
dose for the day. Since there is evidence that therodonts
preferentially break out in the early morning hours, the best time
for treatment is late evening; but if you have confirmed an ich
infestation, do not wait to make the first treatment. The water
change is best done with a gravel vacuum, which will suck up some
of the tomonts and therodonts at the bottom of the tank.
Vegetation and hardscape should be vacuumed as well.
Malachite green plus formaldehyde seems to have little effect on
nitrifying bacteria, but it is wise to monitor ammonium and
nitrite during ich treatment to be sure. It is also the received
wisdom that the mixture is irritating to clown loaches, catfish,
and other scaleless fish. It is sometimes recommended that half
the usual dosage be used on tanks containing such fish, but my
experience is that Kordon's is fine at normal doses with such fish
so long as water changes are made before each dose, and I
think the fish have a better chance against the medication than
they do against the ich. Since such fish tend to be bottom
dwellers, where the heaviest infestation of therodonts is present,
they tend to suffer worst from an outbreak, and aquarium keepers
may be confounding this greater infestation with increased
sensitivity to the medication used to treat it.
Some plants may suffer from the treatment. I have found that
vallisneria tend to slow their growth and staurogyne may suffer as
well. Other species seem to tolerate it well.
Malachite green is easily adsorbed by activated carbon and is
degraded by bright light. You must remove any activated carbon
from your filter before beginning treatment, and, if possible, you
should dim the aquarium lights until treatment is complete. Once
the ich is eradicated, you should reinstall your carbon filters
(or install them if, as recommended earlier, you are avoiding them
in your planted tank.) This will remove the last traces of the
drug.
Treatment is complete when the fish return to their normal behavior and show no white spots for at least three days.
Because copper is highly toxic to many desirable tank
inhabitants, its use should be considered a last resort.
Copper salts, such as copper sulfate, are fairly inexpensive and
are effective against a broad range of pests, including
cyanobacteria, Columnaris, Saprolegnia, Ichthyopthirius,
Oodinium, and snails. Since the toxicity of copper is
quite variable, depending on temperature and pH (much higher at
high temperature and low pH), the recommendation is to start with
the minimum dose of 0.2 ppm copper sulfate, then gradually
increase the concentration in increments of 20% until the
treatment begins to take hold. Obviously, you do not want to use
copper if you have any desirable invertebrates in the tank. Some
fish (such as loaches and scaleless fish) are reputed to be
sensitive to copper, but it is apparently used with success in the
catfish
industry at quite high concentrations. Certain aquatic
plants (particularly Vallisneria and Sagitta) do
not do well with copper at all.
Copper may damage the biological filter, so ammonium levels must
be closely monitored.
Once treatment is complete, the remaining copper must be removed
by repeated water changes, followed by activated charcoal
filtering and the use of a water conditioner advertised as
removing heavy metals.
Methylene blue is from the same family of chemicals as acriflavine, with similar weaknesses and
strengths. It is usually not more effective than malachite green,
and it has a much greater tendency to destroy ammonia oxidizing
bacteria in the biological filter, though it may be easier on fish
and plants.
Nitrofurans are a family of antimicrobials that work by gumming up the DNA of microorganisms. They are effective against a broad spectrum of bacteria and some protozoa, including columnaris and other superficial infections. They are not well absorbed and are sometimes combined with kanamycin, which is.
Quinine is effective against nonresistant strains of ich, and since it is rarely used any more, most strains will be nonresistant. The difficulty is coming by it; it is no longer sold over the counter for cramps in the U.S., as it once was, and it is not a normal part of the pharmacopoeia of most veterinarian clinics. I have not found it in fish shops in years. It may also be effective against Oodinium and other members of kingdom Harosa.
Salt baths are sometimes a useful adjunct treatment for fish that
are under osmotic stress due to injuries or skin lesions. It can
also be curative of some infestations. For example, wild strains
of ich cannot withstand salt concentrations of 5 grams per liter,
while most fish can. However, plants cannot stand salt at this
concentration, so this is a useful strategy primarily for a
hospital or quarantine
tank.
Copyright ©2020 Kent G. Budge. All rights reserved.