Table of Contents
The Chemistry of the Planted Tank
It might be logical to begin with a detailed discussion of photosynthesis. This is a fascinating and intricate process involving most of the chemical elements of life, and understanding it helps us understand the nutritional requirements of aquarium plants. However, the description of so intricate a chemical process seems likely to bog down the reader who is eager to understand the basic chemistry of a healthy aquarium. For that reason, I'll save the detailed discussion of photosynthesis for later.
I'll begin instead with discussing the chemistry of the elements essential for plant life. For aquarium plants to thrive, they need light to provide energy and nutrients to supply the chemical elements of life. These are oxygen, carbon, hydrogen, nitrogen, potassium, phosphorus, calcium, magnesium, sulfur, iron, chlorine, manganese, boron, zinc, copper, molybdenum, and nickel. There may be additional essential elements for some plant species, but they are needed in such tiny quantities that they are never in short supply in a normal aquarium. Tropical aquarium plants also need warmth and a chemically and biologically benign environment.
We can get some idea of the importance of different chemical
elements from the composition of dry plant tissue. These numbers
are for corn plants.
|
Element
|
Corn silage
|
|
|
ppm, dry wt.
|
%, dry wt.
|
|
| oxygen |
450,000
|
45
|
| carbon |
440,000
|
44
|
| hydrogen |
63,000
|
6.3
|
| nitrogen |
13,000
|
1.3
|
| silicon |
12,000
|
1.2
|
| potassium |
9,000
|
0.9
|
| calcium |
2,500
|
0.25
|
| phosphorus |
1,600
|
0.16
|
| magnesium |
1,600
|
0.16
|
| sulfur |
1,500
|
0.15
|
| chlorine |
1,500
|
0.15
|
| aluminum |
1,100
|
0.11
|
| sodium |
300
|
0.03
|
| iron |
90
|
0.009
|
| manganese |
60
|
0.006
|
| zinc |
30
|
0.003
|
| boron |
10
|
0.001
|
| copper |
5
|
0.0005
|
| molybdenum |
1
|
0.0001
|
| nickel |
0.06
|
0.000001
|
Composition likely varies significantly between different plant
species, but this can be taken as a rough guide to what our
aquarium plants are composed of. Some caveats apply. First,
silicon is surprisingly high on the list, but it is not actually
considered an essential plant nutrient. Most species of plants
will grow normally in hydroponics solutions that contain no
silicon at all. However, silicon is the second most abundant
element in the Earth's crust, after oxygen, and a lot of it finds
its way into plant tissues even though it is not essential. There
is some evidence that plants grow better with some silicon
available, but there is practically no likely planted aquarium
setup where there will not be plenty of this element.
The picture is similar for aluminum. Aluminum is the third most abundant element in the earth's crust, and for this reason alone it finds its way into plant tissues. It does not seem to be an essential nutrient for any plant species, but there is some evidence plants do a little better with it. Again, no likely planted aquarium setup will be lacking in this element.
Sodium is the fourth most abundant element in the earth's crust,
and most plants do just fine without it. However, there are a few
plants (the C4 plants) that seem to require it in small
quantities. Other plants can substitute sodium for potassium for
some functions.
It may be interesting to translate these numbers into the numbers
of atoms of each element in a single plant cell. A plant cell has
a mass of about a trillionth of a kilogram. About 90% of this is
water, so the dry mass of a plant cell is one ten-trillionth of a
kilogram. Scientists express these kinds of very large or very
small quantities using scientific notation. In scientific
notation, the dry mass of a plant cell is 1 x 10-13 kg.
The exponent -13 means to shift the decimal places 13
places to the left, so that 1 x 10-13 =
0.0000000000001.
The weight of an individual atom is its atomic weight,
which you can look up easily, divided by one mole. A mole
is a unit of quantity, like a dozen eggs or a ream of paper. It is
defined as exactly 6.02214076×1023 molecules. The number of molecules in a
mole is chosen so that a mole of a particular molecule has a mass
in grams almost exactly equal to the molecular weight of the
molecule. Water has a molecular weight of 18.02518, so a mole of
water molecules weighs 18.02518 grams.
When we go through this exercise for the elements in a single plant cell, we find:
|
Element
|
Atoms per cell
|
| oxygen |
1,690,000,000,000
|
| carbon |
2,210,000,000,000
|
| hydrogen |
3,800,000,000,000
|
| nitrogen |
560,000,000,000
|
| silicon |
26,000,000,000
|
| potassium |
13,800,000,000
|
| calcium |
3,800,500,000
|
| phosphorus |
3,100,000,000
|
| magnesium |
4,000,000,000
|
| sulfur |
2,800,000,000
|
| chlorine |
2,500,000,000
|
| aluminum |
2,500,000,000
|
| sodium |
790,000,000
|
| iron |
97,000,000
|
| manganese |
66,000,000
|
| zinc |
28,000,000
|
| boron |
56,000,000
|
| copper |
4,800,000
|
| molybdenum |
630,000
|
| nickel |
60,000
|
The numbers here amaze me. There are trillions of atoms of the primary elements (carbon, hydrogen, and oxygen) but just 60,000 atoms of nickel in a typical healthy plant cell.Yet those few nickel atoms are essential to keeping the plant healthy.
In addition to the three primary elements, botanists group nitrogen, phosphorus, and potassium together as major nutrients and calcium, magnesium, and sulfur together as minor nutrients. All others are grouped together as trace nutrients. This may seem an odd grouping, but calcium is considered less important than phosphorus because phosphorus is much less available in most environments and so is more important to provide in fertilizers. Chlorine likewise is usually fairly abundant in the environment compared with other trace elements or even with sulfur.
Now let's get into the chemistry of the planted aquarium.
Most of the mass of a live aquarium plant is water, each molecule
of which is composed of an oxygen atom to which two hydrogen atoms
have been bonded. Water is the primary source of hydrogen and
oxygen for green plants. Water is so commonplace, and so obvious a
part of any aquarium, that we tend to take it for granted. But
water is a remarkable chemical substance. To understand why
requires a bit of a "deep dive" into the basic physics of atoms.
An oxygen atom consists of a very small nucleus containing eight protons and (usually) eight neutrons held together by the strong but short-ranged nuclear force. The nucleus is orbited by the eight electrons, which are bound to the atom by the electrostatic attraction between the negatively charged electrons and the positively charged protons in the nucleus. Because there are equal numbers of electrons and protons, the atom as a whole is electrically neutral. (Neutrons have no electrical charge.)
The electrons don't really behave like pointlike particles
following well-defined paths, like planets orbiting the sun in the
solar system. Atoms are so tiny that their behavior is governed by
the weird laws of quantum physics. For the purposes of
understanding aquarium chemistry, we may think of the electrons
(very imprecisely) as clouds of negative charge that have the odd
property that they shrink when they are moving at higher velocity.
The size of the atom is determined by this strange behavior: The
electron clouds cannot orbit any closer to the nucleus than they
do, because that would require them to shrink, implying a higher
velocity that would take them back away from the nucleus.
Electrons repel each other, because like charges repel, while opposite charges attract. Each electron has a spin, which can be taken as either positive or negative. Electrons with like spin experience an additional short-range repulsion that is not present for electrons with opposite spin. The electrons orbiting a nucleus arrange themselves to be as far from each other as possible while being as close to the nucleus as possible; but because of the weaker repulsion between electrons of opposite spin, electrons of opposite spin tend to pair up.
Hydrogen is the simplest of all atoms, with a single electron
orbiting a single proton. The electron behaves a little like a
spherical cloud of negative charge centered on the nucleus, with a
diameter of roughly 100 trillionths of a meter or four billionths
of an inch.
In an oxygen atom, the first pair of electrons fills the space closest to the nucleus — what we call the first electron shell. The remaining six electrons go into the second shell, which has room for eight electrons, arranged in pairs at the corners of a tetrahedron centered on the atom. (This puts the electron pairs as far from each other as possible while remaining close to the nucleus.) Since there are only six electrons remaining, we end up with two pairs of electrons at two corners and one electron each at the other two corners of the tetrahedron.
The corners of the oxygen atom with only one electron behave a little like thin spots in the cloud of negative charge surrounding the positive nucleus. Just a little more of the positive charge of the nucleus can be "seen" through these thin spots. When a lone hydrogen atom approaches a lone oxygen atom, the thin spot attracts the hydrogen's electron and repels its nucleus. Given the chance, the hydrogen's electron will slip into the thin spot, forming a pair with the electron that is already here. But it drags the proton along behind it, so that the pair of electrons simultaneously fills a corner of the second shell of the oxygen and the first shell of the hydrogen. The electrons thus are as close as possible to both nuclei while staying away from the other electrons. This has the effect of bonding the hydrogen atom firmly to the oxygen atom. When a second hydrogen atom likewise bonds to the other corner of the oxygen atom that has only a single electron, you end up with a highly stable water molecule.
What gives water its unusual properties is its tetrahedral
geometry, with protons hanging out on two corners and bare
electron pairs at the other two corners. The protons are attracted
to the bare electron pairs of nearby water molecules. This is
called hydrogen bonding and it causes water molecules to
tend to line up and cuddle close. This gives water an unusually
high melting point and boiling point for such a small molecule.
The fact that a water molecule has two negative corners and two
positive corners also makes water an unusually good solvent for
many substances. The charges help pull molecules of these
substances into solution.
Water also has another very important chemical property: It can
become a source of protons.
The water molecules in a tank of water at a typical tank temperature of 77F (25C) are constantly jostling against each other. Once in a great while, a proton will get knocked loose and stick to an electron pair on a neighboring water molecule:
2 H2O -> OH- + H3O+
The electrons are so strongly attracted to the powerful positive
charge of the oxygen nucleus that they can't normally be pried
away along with the proton. The result is a hydroxide ion,
OH-, with a net negative charge, and a hydronium
ion, H3O+, with a net positive charge.
An ion is a chemically bound group of one or more nuclei and
electrons with a net electrical charge. Chemists reserve the word
molecule for a chemically bound group of two or more nuclei
and electrons with no net charge. Chemists further break down ions
into cations, with a net positive charge, and anions,
with a net negative charge.
Both hydroxide and hydronium, like water, have a pair of electrons at each corner of their tetrahedral second shell, but these molecules differ in whether one, two, or three of these pairs has a proton attached.
Before the nature of the atom was well understood, chemists thought the proton was simply knocked loose:
H2O -> OH- + H+
While we now understand that bare protons cannot exist for any
length of time in liquid water, it is still very common to write
hydronium, H3O+, as if it was just a bare
proton, H+.
Because the extra proton on a hydronium ion is strongly attracted
to the negative charge of a hydroxide ion, if a hydronium ion
bumps into a hydroxide ion, there is a good chance the hydroxide
ion will steal the proton away, converting both hydronium and
hydroxide back to plain water molecules. The two processes, of
water molecules colliding to form hydroxide and hydronium and
hydroxide and hydronium colliding and going back to being water
molecules, rapidly come into balance, producing an equilibrium
between water, hydronium, and hydroxide in which the numbers of
each substance remain steady.
So long as the water is nearly pure, without too much other substances dissolved in it, the rate at which water dissociates depends only on temperature, because the temperature determines how often and how violently the water molecules collide, and there is little for water molecules to collide with except other water molecules. On the other hand, so long as the water is nearly pure, the rate at which hydroxide and hydronium ions neutralize each other depends on temperature and on how much of each ion is present in the water. The more hydroxide is around, the more likely it is that a hydronium will bump into a hydroxide. For the most part, we'll assume from now on that the temperature is 25C (77F), the standard temperature of chemistry, which is conveniently also a fairly reasonable aquarium water temperature. If the two processes are in balance, then
R1 = r2[OH-][H+]
Here R1 is the rate at which water reacts with itself
to form hydronium and hydroxide. Chemists usually express such a
quantity in units of moles per liter per second. The actual value
of R1 is around 1.3 x 10-3 moles per second
per liter. That is, about 8 x 1020 hydronium ions are
created each second in each liter of water (a liter being about a
quart).
We write [OH-] for the concentration of hydroxide in
the water, and [H+] for the concentration of hydronium,
following the ancient and established convention of writing this
as if the proton was floating free instead of part of a hydronium
ion. . The quantity r2 is a rate coefficient
which, when multiplied by the concentrations of hydroxide and
hydronium ions in moles per liter, gives the rate at which
hydroxide and hydronium neutralize each other. This has a value of
about 1.3 x 10-11 liters per mole per second. Neither R1
nor r2 is known very precisely, but this doesn't
actually matter much, because what is important is their ratio:
[OH-][H+] = R1/r2 = 1.0x10-14 M2
Both rates are high enough that this ratio comes into balance
very quickly, within a fraction of a second. We've put the
experimentally measured value on the right. We also use M
as a shorthand for moles per liter. This equation tells us that if
the concentration of hydroxide goes up, the concentration of
hydronium must immediately go down, and vice versa. I've put this
equation in red because it's a good one to remember.
We can also write concentrations in terms of milligrams per
liter, mg/l, and will in some other examples. Since a liter of
water has a mass very close to 1000 grams, a quantity in mg/l is
almost exactly the same as parts per million, ppm, and I will
probably be fairly careless about which I use. They're effectively
the same thing. Expressing reaction rates and equilibrium
conditions in mg/l requires adjusting the numerical value of the
equilibrium coefficient to work with the new units of
concentration. One converts moles per liter to grams per liter
simply by multiplying by the atomic weight.
Now suppose the water is perfectly pure, so that there is nothing
adding hydroxide or hydronium ions to the water except the
dissociation of the water itself. Then it must be the case that
the concentration of hydronium ions equals the concentration of
hydroxide ions. Since the product of their concentrations is
1.0x10-14 M2, this tells us that
there must be 1.0x10-7 M each of hydroxide and
hydronium ions in the water. That's roughly 0.0018 mg/l (or 0.0018
ppm), since a molecule of hydroxide is only a little lighter and
hydronium a little heavier than a water molecule.
This leads us to the first and most fundamental water parameter in the aquarium, the pH. This is defined as:
pH = -log[H+]
In perfectly pure water, the pH is 7.0. Water that has a pH of
7.0 is spoken of as neutral water. Water with dissolved impurities
that give it a higher concentration of hydronium is acidic, while
impure water with a lower concentration of hydronium is alkaline
(or basic). Since hydroxide concentration must go down if
hydronium concentration goes up, acid water has a lower
concentration of hydroxide and alkaline water has a higher
concentration of hydroxide than pure water.
The equation [OH-][H+] = 1.0x10-14
M2 is very reliable. Nothing you can do to the
water that would cause it to violate this relationship for any
length of time would leave your fish and plants alive. Changing
the pH of the water means adding a source of hydronium or
hydroxide to the water. A source of hydronium is called an acid,
while a source of hydroxide is called an alkali or a base.
A surprising fraction of the chemical reactions of life involve
the traces of hydronium found in water. Hydronium participates
directly in the breakdown of many other molecules (a process
called hydrolysis) and it acts as a carrier of electric
charge across biological membranes. Hydronium also reacts with
other substances in aquarium water, modifying how much of each
substance can be found in solution.
Fish and plants tend to like water that is close to neutral, with some fish and plants preferring slightly acid water (down to pH 6.0 or so) and some fish and plants preferring slightly alkaline water (up to pH 8.5 or so.) Yet a drop of pH to 5.0 — dangerously low for most aquarium inhabitants — only requires adding 0.18 mg/l of protons to the water. This is why aquarium water needs some buffering, which means that chemicals are present in the water that tend to soak up excess hydronium or hydroxyl to keep the water at a steady pH. I'll have more to say about that later.
The cheapest way to measure pH, and the most commonly used by aquarium keepers, is a dye indicator. Dye indicators are weak acids, whose molecules have exposed protons that can be snatched away by water molecules:
InH + H2O -> In- + H3O+
where InH is the unaltered (protonated) dye indicator
molecule and In- is the dye indicator molecule minus a
proton (deprotonated). A dye indicator is so called because
one or both of its forms are brightly colored, and the color
changes markedly when the proton is lost. To estimate the pH, you
add a few drops of dye indicator to the water sample being tested,
shake, and compare the color to a color chart. Such a test is
called a colorimetric test.
Each dye indicator has a fairly narrow useful range. This is because the amount of each form is determined by a balance equation of the form
[In-][H+]/[InH ] = ka
where ka, the acid constant, is different for each dye indicator. Equal quantities of both forms of the dye indicator are present when
[H+] = ka
or
pH = -log ka = pKa
It is important to know the pKa of a dye indicator, because the indicator is most useful when the pH is close to pKa. If the pH is just one unit higher, then 90% of the dye indicator is already deprotonated and the solution will have nearly the color of this pure form; if the pH is just one unit lower, then 90% of the dye indicator is in the protonated form and the solution will nearly have the color of this pure form. So there is a narrow range of about 2 pH units where the color is variable enough for the indicator to be useful.
Chemists get around this limitation by combining several dye
indicators, each selected to cover a range of pH, so that the dye
mixture (a universal indicator) displays a rainbow of
colors at different pH. This is not actually necessary for
aquarists, because most aquariums are kept between a pH of 6 and
8. As a result, most aquarium pH testing kits use a single
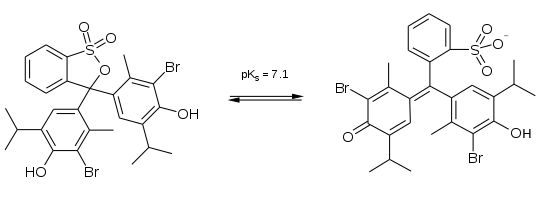
indicator dye called bromothymol blue, which has a pKa
close to 7.1. Bromothymol blue is blue at high pH and yellow at
low pH, turning emerald green (from the combination of blue and
yellow) at neutral pH.

How it works is a little like this: Protonated bromothymol blue has three rings of six carbon atoms (benzene rings) that act a little like conducting loops. The electrons in these rings can oscillate at the right frequency to absorb blue light, which makes the dye look yellow. When bromothymol blue is deprotonated, it's as if an electrical circuit is closed between the benzene rings, allowing electrons to flow between the rings. This roughly halves the oscillation frequency, so that the molecule now absorbs yellow light and the dye looks blue.
You may be scratching your head if you have not studied
molecular structure diagrams before. They will come up many more
times, particularly when we get into the chemistry of
photosynthesis. The molecular structure diagram for a molecule or
ion shows how its atoms bond to each other, using several kinds of
shorthand. Atoms other than hydrogen or carbon are shown with
their symbol, like Br (bromine), S (sulfur), and O (oxygen), and
bonds are shown as lines connecting atoms. Hydrogen is also shown
if it is bonded to any atom but carbon, usually by just putting
the H next to the other atom's symbol. A carbon atom is present
wherever the lines representing bonds meet at a point without an
element symbol. Any hydrogen attached to a carbon is omitted,
since it can be deduced (with practice) just by seeing how many
bonds meet at a point denoting a carbon atom: If there are fewer
than four, then bonds to hydrogen are implied to bring the count
up to four. If you need a more thorough explanation of molecular
structure diagrams, there is a pretty decent longer introduction
starting here.
For saltwater aquarium keepers, or the freshwater aquarium keeper
keeping hard water fish, a slightly higher pH range is desirable,
and high-range pH test kits typically use cresol red with its pKa
of 8.46, This changes color from yellow at low pH to purplish red
at high pH. Cresol red is chemically very similar to bromothymol
blue, but has no bromine and the side chains on its benzene rings
are simpler. This both causes the exposed proton to be a little
more tightly bound (so it takes a higher pH to remove it) and
alters the two oscillation frequencies of the dye.
How reliable are dye indicators? Pretty reliable. They directly measure hydronium concentration, and anything that would cause them to give an erroneous indication would likely also kill everything in your tank. Bromothymol blue is reasonably chemically stable, so that a bottle of prepared indicator solution can remain usable for years, particularly if it is unopened. Keep in mind, though, that once a dye indicator is beyond a unit of pH from its pKa in either direction, its color saturates and you can no longer get a very accurate reading. Bromothymol blue, for example, is pure yellow at any pH below about 6 and pure blue at any pH above about 8.
In principle, you could use an electronic device to precisely
measure the color and get an accurate pH. However, this doesn't
fully solve the problem of the limited range of the indicator. The
more sophisticated approach to measuring pH, used by expert
aquarium keepers, is an electronic pH meter. This uses a pair of
electrodes that respond differently to the presence of hydronium
in the water. The difference in response creates a voltage
difference between the two electrodes that can be very accurately
measured, and once this is calibrated against a solution of known
pH, the meter can continuously monitor pH over a range from about
2.5 to 10.5. This is more than ample for any aquarium containing
live organisms. pH meters can be found online for as little as $13
though, as with most things, you get what you pay for.
Unsuitable pH will affect fish more quickly than plants, so fish
that seem happy and healthy are a good indication that the tank pH
is suitable for your plants as well. Fish have elaborate control
over their internal pH, but when the outside pH gets very far from
the pH they are adapted to, they will be stressed from struggling
to maintain their normal internal pH. Fish that show signs of
stress, such as clamped fins or darting behavior, are an
indication that something is wrong with the water chemistry, and
pH is one of the first things that should be checked.
Fish can often adapt satisfactorily to gradual changes in pH, but
sudden changes are much more stressful. However, there is evidence
that changes of about a unit of pH in the course of a day due to
changing carbon dioxide levels are harmless to most fish, because
this mimics the pH changes fish in the wild experience.
Plants weather pH changes better than fish in the short term, but in the long term, unsuitable pH stunts their growth by interfering with absorption of nutrients. This will manifest as signs of various nutrient deficiencies.
In practice, pH test kits are simple and inexpensive enough that
pH can be monitored regularly rather than relying on the condition
of tank inhabitants to diagnose bad pH.
It may seem a bit strange to talk about oxygen as a plant
nutrient: Don't they extract all they need from water, via
photosynthesis? Yes, but only when they are photosynthesizing!
During the hours of darkness, plants must consume oxygen to
generate energy for their life processes, and non-green parts of
plants (such as roots) must have oxygen at all times. In addition,
we often like to keep fish in our tanks with our plants, and they
need oxygen.
The rate at which oxygen dissolves in water depends on the
concentration in the air. Oxygen also readily diffuses back out of
the water, and this rate depends on the concentration in the
water. So at equilibrium,
r3[O2(g)] = r4[O2(aq)]
where [O2(g)] is the concentration of oxygen in the
room air and [O2(aq)] is the concentration in the
water. The rate coefficients are very poorly known, since they
depend on the surface area of the aquarium, the depth of the
water, and other factors. This is a good example of aquarium
keeping as art; there are simply too many variables for a
precise scientific calculation. Fortunately, we can learn a lot
just by knowing the ratio at equilibrium:
[O2(aq)]/[O2(g)] = r3/r4 = k2
which cancels out all these poorly known factors. k2
is constant at a given temperature, independent of the size or
shape of the aquarium, with a value at room temperature of 0.031.
Room air contains 21% oxygen, or about 0.26 grams per liter. This
means that [O2(aq)] equilibrates to a constant value of
8.2 mg/l in a tank exposed to the normal atmospheric concentration
of oxygen. However, the process of reaching equilibrium is much
slower for oxygen than for pH, and oxygen levels in an aquarium
can vary considerably from the equilibrium value. Plants actively
photosynthesizing will drive the value higher, while fish or
plants that are not photosynthesizing will drive the value
lower. Equilibration is fairly rapid if water is circulating
freely throughout the tank. One of the functions of an aquarium
filter is to help drive water circulation, and additional
circulation can be achieved by running an air stone in the tank.
The water around actively photosynthesizing leaves will soon become saturated with oxygen, resulting in pearling as oxygen comes out of solution and forms bubbles on the surfaces of the leaves. These are welcomed by planted aquarium keepers as an indication that the plants are healthy and active. There are no corresponding bubbles of carbon dioxide around plants in the dark, both because plants slow their metabolism greatly when not exposed to light and because carbon dioxide is much more soluble in water than oxygen.

Most planted aquarium keepers do not do anything special to monitor dissolved oxygen levels. If the fish look happy, the levels are almost certainly adequate. If oxygen levels drop below about 4 mg/l, fish will respond to oxygen stress by acting to reduce their oxygen needs and increase their oxygen supply: They will become much less active, will cluster near the water surface near filter outflows where oxygen is more available, and will gasp for oxygen. These behaviors will be most obvious when the tank is not illuminated. Such behavior requires immediate action to correct the problem. The aquarium keeper must keep in mind that these symptoms also arise from conditions in which oxygen is present but the fish are unable to use it, such as ammonia or nitrite poisoning or infestation with ich.
If other problems are ruled out, then the aquarium keeper must
assume his tank is overstocked for the amount of circulation he is
providing. Either he must increase circulation to get more oxygen
in the tank, or he must remove some of the fish. Circulation can
be increased by turning up the filtration rate (if the filter is
designed to allow this), by adding one or more airstones to
increase circulation in the tank, or by switching to a filter with
greater circulation, such as a power head.
Aquarium keepers who do want an accurate quantitative measure of oxygen levels may purchase dissolved oxygen test kits for around $15 US. There are a couple of ways such a test kit may work. Some use a variation of the Winkler method to determine the oxygen level. This method is based on a reaction between manganese salts and oxygen. A manganese atom has 25 electrons arranged in a rather complicated way around its nucleus. It is easy for manganese to give up two of these electrons to produce the Mn+2 ion. Oxygen is hungry for electrons, and when a reagent solution of manganese (II) sulfate is added to a water sample, the oxygen dissolved in the sample will snatch additional electrons away from some of the Mn+2 ions to form Mn+3 ions. These form a brown precipitate, which is dissolved by adding a second acid reagent. A third reagent is then added that contains a compound called ethylenediaminetetraacetic acid or EDTA.
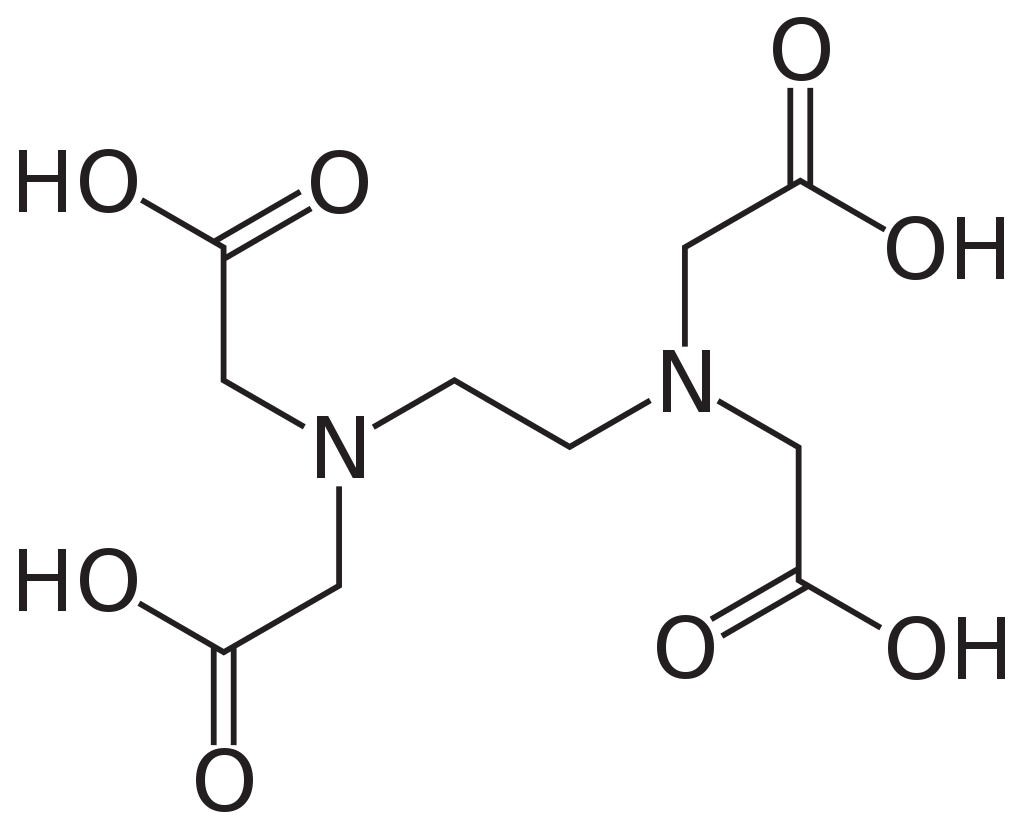
EDTA is an example of a chelating agent. It has four protons that can potentially be snatched away, and at normal aquarium pH, only one will be left. When EDTA encounters a metal ion, the final proton is easily displaced so that all four negatively charged oxygen atoms, plus the two nitrogen atoms, form a kind of cage around the ion:

This binds the ion very tightly, forming a chelate. Chelating agents are so called from the Greek khēlē, "claw", because the chelating agent has the metal ion firmly in its claws! When the ion is Mn+3, the Mn-EDTA chelate takes on a characteristic intense pink color. The intensity of the color is compared to a chart to estimate dissolved oxygen, making this another colorimetric test.
A second form of test kit uses a redox indicator to
measure oxygen. This is similar to a pH indicator, but instead of
the color changing when a proton is snatched away, the color
changes when an electron is snatched away by oxygen. Many of these
are sensitive both to oxygen concentration and pH, complicating
their use, but diphenylamine sulfonic acid is fairly
pH-insensitive and turns from pink to colorless if oxygen levels
drop below saturation.
I have not used oxygen test kits, but I have the impression that the Winkler method is more common.
Both tests are difficult to carry out accurately, since the sample can easily pick up oxygen from room air that throws off the measurement. An alternative is a dissolved oxygen meter, which uses an electronic probe to measure oxygen levels. Dissolved oxygen meters may be purchased on the Internet for as little as $100 US. The less expensive ones operate by measuring the voltage produced when oxygen reacts with a chemical solution in the probe. You will have to purchase the chemicals used in the probe and replenish them as needed. These probes can measure oxygen directly in the tank water, with improved accuracy, but not many aquarium keepers will find this worth the money or fuss.
Water quality is often expressed in terms of the oxidation
potential of the water. This is the difference in voltage
between a bare metal probe placed in the water and an idealized
hydrogen probe in contact with the water via a semipermeable
membrane. Since hydrogen probes are fragile and difficult to work
with, in practice, a silver chloride or SCE probe is used, which
is calibrated against the hydrogen probe. Oxidation potential
represents the tendency of the water to soak up electrons from any
new substance introduced into the water.
Oxygen is hungry for electrons. It is, after all, where we get the term, oxidation, for the process of stealing electrons from other substances. So it is easy to make the mistake of assuming that oxidation potential reflects dissolved oxygen. In fact, in theory, for water that is in complete chemical equilibrium with a reservoir of oxygen, the oxidation potential is
E0 = 1219 + 14.78 log fO2 - 59.2 pH
where E0 is the oxidation potential in
millivolts and fO2 is the partial pressure of oxygen relative to
room air. We calculate a value for rainwater, which has a pH of
5.6, of 887 millivolts. For neutral water in equilibrium with room
air, pH 7, the value is around 804 mV. But this is not what we
actually measure. Rainwater typically has a measured oxidation
potential of around 600 mv, while freshwater aquariums have
typical measured values anywhere from -200 mV to 125 mV.
The explanation is that the oxygen in the air is not in
chemical equilibrium with its surroundings. If it were, our whole
world would be reduced to ashes. (Oxidized, actually!)
Oxygen in air is diatomic oxygen, consisting of pairs of
oxygen atoms clinging rather tightly to each other. It takes a
nudge to get the oxygen to combine with other materials -- a spark
-- and then the oxygen enthustiastically does its thing. So the
dissolved oxygen level and the oxidation potential in almost all
natural waters, and aquariums, are not closely connected and have
to be measured separately.
This is not to say that a low oxidation potential does not point
to low dissolved oxygen. There is a connection. But the two are
not related in any simple way in the real world. If your
aquarium is at an oxidation potential of -200 mV, that corresponds
to a complete absence of any dissolved oxygen at all in
equilibrium. Fortunately, the aquarium is not in equilibrium; but
something is present that has a strong tendency to soak up oxygen,
and that's going to compete with your fish and plants for the
oxygen supply.
Most of the dry mass of an aquarium plant is composed of large molecules built around a backbone of linked carbon atoms, or of nitrogen atoms alternating with pairs of carbon atoms, to which other atoms or groups of atoms are attached. For example, carbohydrates make up most of the dry weight of plants, and are composed of carbon chains or rings to which hydrogen and oxygen are attached. Vegetable fats are mostly carbon chains to which hydrogen is attached, while protein is chains of nitrogen alternating with pairs of carbon to which a great variety of molecular groups can be attached. It is the ability of carbon atoms to link together in complex ways that makes life possible.
A carbon atom has six protons and (usually) six neutrons in its nucleus, with six orbiting electrons. As with oxygen, two of the electrons fill the innermost shell. The other four each occupy a corner of the tetrahedral second shell. If another carbon atom approaches, it is easy for electrons in the corners of the two atoms to pair up, being shared by both atoms. Either one, two, or three electron pairs are shared by the two atoms (though the later is very rare in biological compounds.) We speak of these as single, double, or triple bonds. The remaining corners of the carbon atoms are free to bond with other atoms, including more carbon atoms.
While plants can get plenty of hydrogen and oxygen from the water in the aquarium, they usually must get their carbon from carbon dioxide dissolved in the water. They do this by capturing energy in the form of light falling on their leaves, and use this energy to split water molecules into oxygen and hydrogen. The oxygen is released back into the aquarium water, and the hydrogen and additional captured light energy are used to convert carbon dioxide and additional water molecules to simple carbohydrates. These can then be used as building blocks for more complex compounds. We'll revisit this photosynthesis process in considerably greater detail later on.
The more carbon dioxide that is dissolved in the water, the more
that is available for your plants. However, too much is harmful to
fish, which are trying to get rid of carbon dioxide in their
bloodstreams, produced by metabolizing the food they eat. How much
carbon dioxide can we expect to find in a tank in equilibrium? The
ultimate source of carbon dioxide in a low-tech aquarium is the
carbon dioxide present in the air in the room where the aquarium
is placed. The amount of carbon dioxide in air varies from place
to place and over the course of a day, but it averages about 387
parts per million, or 0.0387%. It will be less in the canopy of a
forest on a sunny spring day, and more in a large city late in the
evening, but we can take 387 parts per million as a reasonable
average value. Given the low density of air (about 1.26 g/liter),
this is about 0.49 mg/liter of carbon dioxide in room air.
Carbon dioxide readily dissolves in water, and the concentration
in equilibrium with room air follows the same kind formula as for
oxygen:
[CO2(aq)]/[CO2(g)] = k2
k2 for CO2 has a value at room temperature
of 1.189. This means that [CO2(aq)] equilibrates to a
constant value of 0.58 mg/l in a tank exposed to the normal
atmospheric concentration of carbon dioxide — not very much carbon
dioxide, but more than the concentration in room air.
A carbon dioxide molecule consists of two oxygen atoms double-bonded to a carbon atom. Unlike water molecules, which can react with each other to establish pH, carbon dioxide molecules do not react with each other in any important way. However, dissolved carbon dioxide does react with water to change the pH. When a carbon dioxide molecule collides with a water molecule, if the impact is forceful enough, there is a chance one of the double bonds on the carbon atom will partially break. This causes the electrons around the two molecules to do a bit of shuffling, and when the dust settles, we have a single molecule of carbonic acid, H2CO3. Carbonic acid is not stable, and soon falls apart into water and dissolved carbon dioxide again. This sets up yet another equilibrium:
[H2CO3]/[CO2(aq)] = k3
In other words, the amount of carbonic acid depends on the amount
of dissolved carbon dioxide. At normal temperature, this
equilibrates to less than two carbonic acid molecules for every
thousand dissolved carbon dioxide molecules (k3 = 1.7 x
10-3). This value is not actually known very precisely,
but this will turn out not to matter much.
Like all acids, carbonic acid can donate a proton to a water molecule to produce hydronium. The deprotonated carbonic acid is called bicarbonate, HCO3-:
H2CO3 + H2O -> HCO3- + H3O+
Bicarbonate is itself a very weak acid, because it can donate a second proton to the water to produce carbonate, CO32-. However, bicarbonate and carbonate also tend to grab back their protons, resulting in additional equilibria:
[HCO3- ][H+]/[H2CO3] = k4
[CO32- ][H+]/[HCO3-] = k5 = 5.0 x 10-11 M
It turns out that little carbonate is present at normal aquarium pH (less than 1% of of the amount of bicarbonate when the pH is below 8.3), so we normally only need worry about bicarbonate. Also, since carbonic acid concentration depends on carbon dioxide concentration, chemists tend to substitute [H2CO3*] = [H2CO3] + [CO2(aq)] in their equations, to yield
[HCO3- ][H+]/[H2CO3*] = k6 = 4.25 x 10-7 M
These are not actually the most useful units for this expression. We take the log of this formula and substitute log[H+] = -pH and fCO2 = [H2CO3*]/1.31 x 10-5 M, the concentration of carbon dioxide relative to normal room air, and shuffle units and take logarithms to get
log [HCO3- ] = pH + log fCO2 - 11.25
This tells that, if our tank is equilibrated with room air (log
fCO2 = 0) then pH must go up when the concentration of
bicarbonate goes up, and vice versa.
Do we actually care about bicarbonate? Not so much. Plants can't use it directly, though it forms a kind of reserve of dissolved carbon that can be converted to carbon dioxide. We are more concerned with how much carbon dioxide is in our water, fCO2. This is often well out of equilibrium with room air, due either to plants consuming carbon dioxide or plants and fish producing carbon dioxide. We have a connection now between this quantity and pH, but we're only halfway there unless we also can determine the concentration of bicarbonate.
Suppose we are working with water that is absolutely pure except for some dissolved carbon dioxide. The tank will contain water, hydronium, hydroxyl, dissolved carbon dioxide (including carbonic acid, which we will always lump with dissolved carbon dioxide from now on), and bicarbonate. The only source of hydroxyl is water dissociating, while the only source of bicarbonate is carbon dioxide reacting with water. Both reactions produce hydronium. It must be then case then that
[H+] = [HCO3- ] + [OH-]
We take the acid-base equilibrium between [H+] and [OH-]
and the equilibrium relationship between bicarbonate, hydronium,
and carbon dioxide saturation, and after some algebra, we obtain
fCO2 = 1.78 x 1011 (10-2pH - 1 x 10-14)
That's a bit awkward to use, but it tells us (1) that pure water
exposed to air can never have an alkaline pH, and (2) pure water
in equilibrium with room air will have a pH of about 5.6. The
carbon dioxide in air is enough to make pure water more acidic
than is comfortable for most fish. (But water that pure is going
to be hard on fish for other reasons as well.) The bicarbonate
concentration will be about 2.4 x 10-6 M or 0.14 mg/l.
Keep in mind that this equation only applies to pure water; it is
not a general relationship for typical impure tank water.
We never actually use pure water in a fish tank. It always has some dissolved carbonate salts in it, and these make a very big difference.
Suppose we add 17.848 mg/l of calcium carbonate, CaCO3,
to pure water. (The oddly precise value is chosen for a reason.)
It will take some time, but this small amount of calcium carbonate
will eventually dissolve in the water, in the form of calcium
ions, Ca2+, and carbonate ions, CO32-.
Almost all the carbonate will recombine with some of the hydronium
in the water to form bicarbonate, some of which in turn will
recombine with more hydronium to form carbonic acid, most of which
will break down to dissolved carbon dioxide, some of which will in
time diffuse out of the water. We know that the positive charges
in the tank come from [Ca2+] and [H+] and
the negative from [HCO3- ] and [OH-],
and these must balance:
2[Ca2+] + [H+] = [HCO3- ] + [OH-]
(We are again ignoring [CO32- ], negligible at normal freshwater tank pH.) The quantity of calcium ion is not going to change unless we do other things to the tank, so we can take it as fixed at 7.146 mg/l, the amount we added. In terms of actual numbers of ions, this is 1.783 x 10-4 moles/liter. We need two more equations relating the other three quantities; these are
[OH-][H+] = 1.0x10-14 M2
and
[H+] = 5.62 x 10-12 M2 fCO2 / [HCO3- ]
When we work through the algebra, we end up with
[H+] = [Ca2+](sqrt(1 + 5.62 x 10-12 fCO2 /[Ca2+]2) - 1)
[HCO3- ] = 2.25 x 10-11 fCO2 / [H+]
Unless the calcium concentration is extremely low or the carbon dioxide saturation is extremely high, this is approximately
[H+] = 2.78 x 10-12 fCO2 / [Ca2+]
[HCO3- ] = 2[Ca2+]
For our example, [HCO3- ] = 3.57 x 10-4M
or about 21.76 mg/l of bicarbonate. The pH is about 7.8. We see
that adding a small amount of calcium carbonate has greatly
increased the pH, by about 2.2 units. It has also buffered
the pH: If we add another 17 mg/l of calcium carbonate, the pH
goes up by just 0.3 units, to 8.1. Buffering helps hold the
pH steadier and is vital in a healthy tank. Bicarbonate added to
water as soluble hydroxide, carbonate, or bicarbonate salts is
referred to as carbonate hardness. In the case of added
hydroxide, the water actually absorbs atmospheric carbon dioxide
to come into equilibrium, but the effect is the same as if the
carbonate was added directly.
In our example, the molar concentration of bicarbonate is almost exactly twice the concentration of calcium ions. This approximation holds for reasonable concentrations of calcium carbonate and CO2 levels. More generally, the concentration of bicarbonate will nearly equal the total concentration of positive charge added as carbonates, bicarbonates, or hydroxides, Thus, adding sodium carbonate instead of calcium carbonate will yield a millimole of bicarbonate for every millimole of sodium ion, and some aquarists use sodium bicarbonate to increase the carbonate hardness of very soft water. If carbon dioxide injection raises the dissolved carbon dioxide concentration by a factor of ten, the pH drops, but the bicarbonate concentration holds steady. It's possible — I am speculating here; this is not confirmed science — that the tendency of bicarbonate concentration to hold steady with varying CO2 levels in spite of the corresponding change in pH is why fish seem able to handle moderate pH swings if they are due to changes in CO2 level. Bicarbonate is the main buffer in the bloodstreams of fish, and a ratio of external to internal bicarbonate that holds steady may be protective against pH swings.
We now have a connection between the amount of carbon dioxide in
the tank, the pH of the water, and the carbonate hardness. We also
know that some carbonate hardness is desirable, because it smooths
pH fluctuations. All that is needed is a succinct formula
connecting these quantities in useful units. pH and fCO2
are reasonably useful, but we will choose German degrees of
carbonate hardness, for which the symbol is dKH, as our
measure of water hardness. This is a unit widely used in the
aquarium business. A degree of carbonate hardness is defined as
the hardness produced by 17.848 mg/l of calcium carbonate, which
is why we chose this oddly precise value for our example. This odd
definition in turn comes from 17.848 mg/l of calcium carbonate
having exactly as many calcium ions as 10 mg/l of calcium oxide. A
degree of carbonate hardness is then equivalent to 3.566 x 10-4
moles/liter of positive charges added as part of hydroxide,
carbonate, or bicarbonate salts.
log fCO2 = log dKH - pH + 7.81
This is the equation that really matters for an aquarium keeper.
It relates the German degrees of carbonate hardness, dKH, to the
pH and the concentration of carbon dioxide in the aquarium
relative to the equilibrium value with normal room air, fCO2.
Our equation tells us that water with a hardness of 4 dKH in
equillibrium with room air will have a pH of about 8.4. If the pH
is below this value, then there is excess carbon dioxide in the
tank; if it is above this value, then the tank has somehow become
depleted in carbon dioxide, perhaps from active photosynthesis.
This formula breaks down for very low or very high values of dKH,
but is accurate for values of dKH common in planted aquariums.
The most common carbonate hardness test kits measure dKH by
having you add drops of a test reagent to a measured sample of
water, one drop at a time, until the color changes. The indicator
solution is a mixture of dilute hydrochloric acid and bromothymol
blue. Hydrochloric acid is a solution of hydrogen chloride in
water, and hydrogen chloride is strong acid consisting of a
hydrogen atom bonded to a chlorine atom. It takes very little
encouragement to get the proton from the hydrogen atom to come
loose and attach itself to a nearby water molecule, and the
resulting chloride ion has almost no inclination at all to snatch
the proton back, so adding hydrogen chloride to water is
practically the same thing as adding hydronium to the water. Each
drop of hydronium-rich test solution converts some of the
bicarbonate in the sample back to carbon dioxide, which uses up
the added hydronium. The corresponding amount of positive charge
is now balanced by chloride rather than bicarbonate. When the
bicarbonate is all replaced by chloride, the hydronium
concentration shoots up, dropping the pH and changing the color of
the dye indicator. The test is typically calibrated so that each
drop of test solution consumes one dKH of carbonate hardness. Such
a test is called a titration test.
How reliable is the test? Not very if the hardness is low. If the
color changes on the first drop, all you know is that your
hardness is less than 1 dKH. If it takes ten drops, you get a
pretty good estimate that your dKH is 10, accurate to around 5%.
Another concern is under what conditions you do the test.
Carbonate hardness is usefully tested only in water that has had
any excess carbon dioxide flushed out. If you take water out of a
tank that has carbon dioxide injection, and do not take the time
to let it equilibrate with room air, the low pH due to all the
carbonic acid in the water will mask the presence of the
bicarbonate. The reading will mean little; the indicator will
saturate on the first drop even when there is quite a lot of
carbonate hardness in the water. Also, because hydrochloric
acid is volatile, the test solution slowly loses its potency once
the bottle is open. It should be discarded after a year or two.
Many aquarists have reported that the pH of their tap water is high for the measured dKH, and drops as the tap water is allowed to equilibrate with room air. This tells us the municipal water coming out of the tap is depleted in carbon dioxide. How can this happen? It's likely a result of water treatment that raises the pH of the water by deliberately adding some alkali to it. It's not so much that the carbon dioxide has been depleted as that the hardness has been augmented, but the effect is the same. This is done to encourage a thin film of calcium carbonate to form inside metal pipes, reducing the risk of heavy metals (particularly lead but also copper) dissolving and making the water unhealthy to drink. Under aquarium conditions, it is unlikely that calcium carbonate will ever precipitate, but we'll explore that possibility later on in these notes.
In the case where you really need to precisely measure a low dKH, you can use a larger sample size than the test kit calls for to increase sensitivity. If the sample is ten times larger than the kit calls for, a single drop of reagent will consume only a tenth of a degree of hardness instead of one degree of hardness. It may be difficult to make out the color change at these dilutions, but you can compensate for that by adding additional bromothymol blue from a pH test kit. Add no more than is needed to see the color change.
Alternately, you can make sure the water sample is well
equilibrated with room air (log fCO2 = 0), measure the
pH, and use the equation: log dKH = pH - 7.81. This will work well
for fairly low values of dKH, so long as your indicator is in its
useful range. For bromothymol blue, this means from about 6 to
about 8. That corresponds to a hardness between 0.015 dKH and 1.54
dKH. You really do need the carbonate hardness test kit to measure
carbonate hardness in all but very soft water.
A value often recommended for planted aquariums is 4 dKH, which
is somewhat on the alkaline side unless carbon dioxide is being
injected into the tank. (I'll discuss that presently.) A value
less than 2 dKH is risky in an injected tank for many fish
species, since normal carbon dioxide target levels will drop the
pH below 6.5. If KH is too low, it can be increased by adding
limestone rocks or crushed coral to the tank, which will slowly
dissolve, or by adding sodium bicarbonate (baking soda) to
replacement water. The latter method is more easily controlled.
Addition of 66 mg of sodium bicarbonate per gallon of water will
raise KH by 1 dKH. One can make up a solution of 33 grams of
baking soda in 500 ml (about a pint) of distilled water, and add
one ml of this solution to each gallon of water whose KH is to be
raised. I will often be giving recipes for such stock
solutions, because you can purchase medicine syringes for
measuring out milliliter quantities of liquids in practically any
pharmacy, and it is much easier to precisely measure out (say) 33
grams of baking soda for making up a stock solution than measuring
out less than a gram of baking soda to put directly into the
aquarium.
If excess sodium is a concern, you can substitute very finely powdered calcium carbonate at a rate of 72 mg per gallon. Again, one can make up a stock solution of 36 grams powdered calcium carbonate in 500 ml water and add one ml of this solution to each gallon of water. Since 36 grams is far more calcium carbonate than can be dissolved in a half pint of water, the stock solution must be shaken thoroughly to mix the powder with the water before measuring out the treatment dose. Once diluted in the aquarium, the powder will slowly dissolve.
dKH can also be too high, so that the water is uncomfortably
alkaline for some fish and plants. The safest solution is to use a
different source of water. One can dilute alkaline tap water with
deionized water, either purchased by the gallon from the grocery
store or produced by reverse osmosis (RO) from a home system. This
will also lower the total hardness, which we'll discuss later on.
If you have water with suitable total hardness but too high a
carbonate hardness, the KH can be reduced chemically by 1 dKH by
adding 200 mg of sodium bisulfate to each gallon of water.
Commercial products for reducing alkalinity of swimming pool water
are usually based on sodium bisulfate, as are pH lowering products
sold for aquariums. Remember that, for a tank in equilibrium with
air, lowering pH is equivalent to lowering dKH. One can make up a
solution of 100 grams of sodium bisulphate in 500 ml of distilled
water, and add one ml of this solution to each gallon of water
whose KH is to be lowered by one degree. Be aware that sodium
bisulfate is highly corrosive and avoid direct contact with skin
or clothing. If excess sodium is a concern, and if you are
experienced working with strong acids, KH can also be reduced by 1
dKH by adding 175 mg of concentrated hydrochloric acid (30%
concentration, available in hardware stores as "muriatic acid") to
each gallon of water. One can make up a solution of 88 ml of 30%
hydrochloric acid in 500 ml of distilled water, and add one ml of
this solution to each gallon of water whose KH is to be lowered.
Although concentrated hydrochloric acid is dangerous if
mishandled, it is harmless once diluted in the tank, where its
only residue is innocuous chloride ion. The stock solution is also
much safer to handle than the concentrated hydrochloric acid. Safe
handling procedures for strong acids can be found here.
Water that has been chemically treated to lower dKH will likely test quite acidic at first, but its pH will slowly rise as it re-equilibrates with room air. For each degree of KH neutralized, there will be an excess of 8 ppm of carbon dioxide in the freshly treated water. If the water was initially at 5 dKH, this means the pH will briefly drop from its initial value near 8.5 to 7.2. If you are injecting CO2 in your aquarium, the treated water may already be close to your target pH and CO2 concentration. Otherwise, you will need to let it aerate overnight to reach a stable pH. Running an airstone in the treated water overnight can help flush excess CO2 and bring the pH to its stable value.
A more gradual way to lower dKH that does not require working with corrosive chemicals is to add a source of tannic acid to the tank. This usually means adding driftwood, almond leaves (available at some aquarium shops), or filtering the water through aquarium peat. Tannic acid will slightly discolor the water but can lower dKH substantially. Tannic acid also acts as a buffer, though with a pKa around 6, it is most effective as a buffer in a somewhat acid tank. This is more appropriate for acid water fish such as discus or ram cichlids than for most planted aquariums.
Aquarium plants will have no lack of hydrogen and oxygen so long
as there is light energy for photosynthesis to break up water
molecules. However, their growth can be limited by the
availability of carbon dioxide. With just 0.58 mg/l of carbon
dioxide dissolved in water in equilibrium with room air, the
carbon dioxide dissolved in aquarium water is rapidly depleted.
The larger reservoir of dissolved carbon is bicarbonate ion, but
this must first recombine with hydronium in the water to form
carbonic acid, which in turn must decompose into water and carbon
dioxide. Different plant species have different strategies for
gathering up as much carbon dioxide as possible, but all include
an enzyme called beta-carbonic anhydrase. Enzymes
greatly speed up specific chemical reactions, so they are highly
selective catalysts. Beta-carbonic anhydrase speeds up the
conversion of bicarbonate and hydronium to water and carbon
dioxide.
Beta-carbonic anhydrase is formed from a strand of protein wound
around a zinc ion, which is the prosthetic group or
"cutting edge" of the enzyme. This tells us that zinc is required
for healthy plant growth. I'll describe other aspects of carbon
dioxide uptake by plants in the next
chapter.
Even the bicarbonate reservoir will be insufficient if the carbon dioxide absorbed by the plant is not continually replenished. Vigorous aeration of a tank will allow atmospheric carbon dioxide to dissolve in the water, and water circulation will carry the carbon-rich water past the plants. However, plant growth can be greatly enhanced by injecting additional carbon dioxide directly into the tank, thus raising fCO2 far above the value in equilibrium with room air. One study found that natural waters have a carbon dioxide value that averages around 1.7 mg/l, three times the equilibrium value with the atmosphere. Laboratory experiments indicate that aquatic plants can benefit from levels that are higher still, as much as 30 mg/l, as long as lighting and other nutrients are adequate.
As a result, carbon dioxide injection has become very popular for
planted aquariums. This consists of providing a carbon dioxide
supply to some kind of dispersal mechanism that dissolves carbon
dioxide in the aquarium water.
There are three ways to supply carbon dioxide to an injection system that are in common use today.
The simplest, but least satisfactory, way to generate carbon
dioxide is to mix yeast with sugar and allow the yeast to ferment
the sugar to alcohol and carbon dioxide. Table sugar, or sucrose,
consists of two simpler sugars, fructose and glucose, linked
together. The yeast cell first splits the sucrose into the simpler
sugars. Each simple sugar, which is built on a chain of six carbon
atoms, is then split into two three-carbon molecules of a compound
called pyruvate. Finally, each pyruvate molecule is split into a
carbon dioxide molecule and a molecule of ethanol. The yeast cell
obtains a small amount of energy from this process, enough to keep
it alive.
For yeast to generate carbon dioxide at a useful rate, it must be kept away from oxygen. If oxygen is available, the yeast will convert the sugar completely to carbon dioxide without producing any ethanol (the Pasteur effect.) This produces three times as much carbon dioxide from a given quantity of sugar as fermentation without oxygen. However, this also yields fifteen times as much energy for the yeast to use, so the yeast is in no rush to consume all the sugar. In other words, keeping oxygen away from the yeast causes it to waste two-thirds of the sugar, but it also causes the yeast to generate carbon dioxide much more rapidly.
The setup is simple. One part sugar is added to three parts water
and dissolved. Half a tablespoon of yeast is then added to each
liter (roughly a quart) of sugar solution. Some baking soda is
also added to the solution; the amount can be adjusted with
experience, but a teaspoon per liter is a good staring point.
Baking soda raises the pH, which slows the fermentation but allows
it to continue longer. This gives you a steady flow of carbon
dioxide for about a week rather than a rapid burst of carbon
dioxide that is gone in a couple of days. The mixture goes in an
old two-liter soft drink bottle, which is tightly capped. Aquarium
tubing is run through a hole in the cap (sealed with aquarium
silicone) and to the dispersal system.
The catch is that there is no way to stop the fermentation once started, short of killing the yeast. This means that much of the carbon dioxide must be wasted during times that the tank is not illuminated. For a typical photoperiod of 8 hours a day, that's two-thirds of the carbon dioxide wasted. There is also very little control over the rate of generation, and it takes some time — up to an hour — for a fresh batch of yeast to begin producing ample carbon dioxide. This makes yeast fermentation a fairly unsatisfactory way to generate carbon dioxide in the long term.
This is a purely chemical process for generating carbon dioxide.
Baking soda is mixed with an equal amount of water in one bottle;
a moderately weak acid solution is prepared in a second bottle.
The two bottles are connected by tubing arranged in such a way
that squeezing the acid bottle squirts a good jolt of acid
solution into the baking soda bottle. The baking soda is a source
of bicarbonate ion which reacts with the hydronium in the acid
solution to produce carbonic acid, which decomposes to carbon
dioxide and water. The carbon dioxide goes out a second tube to
the dispersal system. The tube in the acid bottle is arranged such
that when pressure drops in the soda bottle, more acid is forced
through the tubing into the soda bottle. You can find preassembled
tubing sets for sale on the Internet for a few dollars. These
screw onto two used 2-liter soft drink bottles. The sets typically
include a cheap pressure gauge and a screw valve, and a magnet for
pulling the tube in the acid bottle out of the acid solution to
stop the reaction if necessary. With a little practice, you can
get the hang of adjusting the system so that carbon dioxide is
generated fairly uniformly during the day and can be turned off at
night. This is far less wasteful than a yeast generator, it can
produce a large volume of carbon dioxide much more rapidly, and
the reaction can be better controlled.
Any acid solution can be used, but the reaction is easier to control if a well-buffered acid is used. Vinegar is inexpensive but unbuffered and so it is harder to keep the reaction steady. The usual choice is citric acid, with one part dissolved in three parts of water. Citric acid is not a normal grocery item, like vinegar, but it can be purchased easily online in bulk. It makes a much more acidic solution than vinegar. Better yet, it is highly buffered, because the citric acid is a triprotic acid. This means it has three easily detached protons to react with water to form hydronium. The last of these does not come loose until the solution is almost neutral in pH. The result is that when a squirt of citric acid goes into the soda bottle, the first proton comes loose at once, reacting with the bicarbonate to make a great fizz of carbon dioxide. As this gradually leaves the bottle for the injection system, the pH of the solution in the soda bottle rises. This allows the second proton to come loose, producing more carbon dioxide to replace that which has been used. Further loss of carbon dioxide raises the pH still higher, allowing the final proton to come loose and generate a final burst of carbon dioxide.
Acid/soda generation is a lot of work to maintain, but it is a good way to practice carbon dioxide injection, see the benefits, and decide whether to invest in a much more costly pressurized carbon dioxide system.
The best system in the long run is pressurized carbon dioxide. This cuts to the chase and supplies pure carbon dioxide from a cylinder that contains liquefied carbon dioxide under high pressure. A cylinder containing five pounds of liquefied carbon dioxide can last many weeks (depending on how much you are injecting) before needing to be recharged, and most cylinders can be recharged for significantly less than it costs to purchase sugar or acid and soda producing an equal quantity of carbon dioxide. In my area, I can refill a five pound carbon dioxide cylinder for $20, or $4 per pound of carbon dioxide, while the citric acid and soda costs $5.50 per pound of carbon dioxide. The catch is that the starting cost of the system is considerably more expensive — up to $300 for a basic system consisting of the pressure regulator and other hardware and a pair of cylinders.
At a minimum, the system requires a pressure regulator to bring
the very high pressure in the cylinder down to a pressure low
enough for aquarium tubing; a solenoid to open and close the
cylinder electrically; and a couple of pressure gauges to show the
pressure on each side of the regulator. Fancier systems may
include an electronic control that not only turns off the system
at night, but turns it off if a pH meter in the tank shows that
the pH is dropping too low.
Although the regulator drops the pressure of the carbon dioxide by a large factor, the low pressure side is still at a significantly higher pressure than a do-it-yourself yeast or acid/soda system. This gives more options for the dispersal system.
Carbon dioxide becomes liquid at room temperature (25C or 77F) at
a pressure of 6440 KPa or 934 PSI. (Cylinders are typically rated
at 1800 PSI, so there is little danger of overpressurizing a
cylinder.) The pressure remains very close to 934 PSI until almost
all the liquefied carbon dioxide is used. Once it is gone, the
pressure drops relatively rapidly. Inexpensive regulators,
described as single stage regulators, are prone to
actually increase the pressure on the low pressure side when the
cylinder pressure drops. This is known as an end of tank
dump and it can cause a burst of carbon dioxide injection
that can kill every fish in the aquarium. Some aquarium keepers
never see an end of tank dump; others report seeing it every time
the cylinder runs low.
End of tank dump can be avoided one of four ways. The first is to
be scrupulous about changing the cylinder as soon as the pressure
drops below about 600 PSI, indicating the liquid carbon dioxide is
gone. This approach can work, but a few days' inattention is all
it takes for disaster. It is also problematic for leaving the
system on a timer while on vacation.
The second approach is to put some kind of pressure relief mechanism on the low pressure side of the system to ensure that pressure above a danger threshold is vented to the room instead of the aquarium. Relief valves for moderate pressures can be found online and are not expensive. If you normally work with a low side pressure of 20 PSI, a 30 PSI relief valve should reliably vent any end of tank dump before it can do significant harm. For very low pressure dispersal systems. one can even rig a DIY pressure relief system. This consists of a larger diameter line or multiple smaller diameter lines connected to your main dispersal line, and run into a vertical pipe (PVC works fine) capped at the bottom and filled with water to a depth of several feet. This will vent any pressure that is much higher than that going into your tank. The chief drawback of pressure relief valves, commercial or DIY, is that they can start venting small amounts of carbon dioxide at working pressure. In effect, they can act as leaks in the system, wasting carbon dioxide.
The third method is to put the solenoid on a control that
monitors pH. If there is an end of tank dump, this will detect the
drop in pH close the solenoid. However, if you want to invest that
much in your system, you might as well go with the fourth
solution.
The fourth and surest method is to buy top-quality equipment. This will naturally be considerably more expensive than a simpler system. A two-stage regulator is actually two regulators in series, which drop the cylinder pressure in two stages (hence the name). Two-stage regulators are almost immune to end of tank dumps and the pressure on the low pressure side is more stable. A two-stage regular should not be confused with a two-gauge regulator, which is typically just a single stage regulator with pressure gauges on both sides. This is useful as far as it goes, but it is not a two-stage regulator. Another possibility is a down-stream regulator, which regulates based on low-pressure side pressure rather than cylinder pressure. Even a high-quality, well-designed single stage regulator gives considerable protection against an end of tank dump.
Whatever source of carbon dioxide is used, it must be supplied to
a dispersal system through a needle valve or some other device
that allows the amount of carbon dioxide flowing into the system
to be controlled. It is also a good idea to run the carbon dioxide
through a check valve, particularly in a pressurized system.
Carbon dioxide is so soluble in water that, when the solenoid is
closed at night, the carbon dioxide left in the tubing can
dissolve in the water, drawing the water up into the tubing and
potentially wrecking the regulator. The check valve prevents this
back flow.
Most systems also have a so-called bubble counter between the
needle valve and the dispersal system, which allows you to see how
many bubbles of carbon dioxide are being released into the
aquarium each minute. This is often a single unit with the check
valve.
This is an arrangement of plastic baffles into which the carbon dioxide is bubbled from the end of the supply tubing. Each bubble must slowly work its way up the ladder, which gives the time needed for most of the bubble to dissolve. If arranged well, you will see large bubbles go into the ladder and much smaller bubbles come out the top. It is possible to dissolve better than 80% of the carbon dioxide in this manner, with the rest rising to the top of the aquarium and being lost to the room. Bubble ladders are also suitable for both high and low pressure carbon dioxide. However, bubble ladders can handle only a limited flow of carbon dioxide, they tend to accumulate algae, they take up a fair amount of tank space, and many aquarium keepers find them unsightly. For this reason, they are a do-it-yourself project; I have not seen bubble ladders on sale on the Internet in some time.
A ceramic diffuser is a plate of porous ceramic or spun glass
into which the carbon dioxide is piped. The carbon dioxide bubbles
up through the fine pores in the plate, producing a stream of
relatively small bubbles that dissolve fairly rapidly in the tank
water. As with a bubble ladder, some of the carbon dioxide is
bound to reach the top of the aquarium and escape. However, a
ceramic diffuser works adequately over a broader range of flow
rates than a bubble ladder.
Some diffusers produce smaller bubbles than others. This may actually be counterproductive. When one observes bubbles rising from a fairly coarse diffuser, it is striking how the larger bubbles rise very quickly at first, then abruptly slow as they drop below a critical size. The smaller bubbles rising more slowly also appear to dissolve much more slowly, usually making it to the surface of the water. (Fortunately, by then, most of the carbon dioxide has dissolved, so wastage is not significant.) This seems counter-intuitive, since a smaller bubble has a higher surface to volume ratio. It may be an effect of surface tension (greater in a smaller bubble), of the more rapid movement of the larger bubble, or of residual gases less soluble than carbon dioxide being left in the smaller bubble. Certainly a freshly recharged yeast or acid/soda generator will have considerable inert air in its output until the lines are flushed, but this does not seem to explain the phenomenon, which seems as noticeable when the charge is almost exhausted as in a fairly freshly charged system. More research is required to understand it.
Some diffusers are deliberately designed to release a mist of
very small bubbles and are intended to be positioned so that tank
circulation distributes this mist throughout the tank. The carbon
dioxide is thus distributed to the aquarium plants in gaseous
rather than dissolved form. This is claimed to allow a greater
amount of carbon dioxide to be injected without affecting fish.
The catch is that the mist is clearly visible in the aquarium
water.
As shown in the photograph, low-pressure diffusers run at low
flow rates can acquire a film of green algae after a time. This
rarely seems to hinder the operation of the diffuser, but can be
removed periodically by soaking in bleach or hydrogen peroxide.
The latter leaves no harmful residues when the diffuser is
replaced in the tank. In general, disinfection of any tank
equipment with bleach should be followed by a rinse in
dechlorinator to ensure no traces remain. I'll discuss this later
in more detail.
A carbon dioxide reactor is used primarily as part of an external
filtration system that pipes water out of the tank, filters it in
an external canister, and returns the water to the tank. The water
is run through a device that deliberately generates turbulence and
into which the carbon dioxide is injected. The turbulence helps
break up the injected bubbles and dissolve them in the water. Such
systems are claimed to be nearly 100% efficient and the entire
injection system can be kept outside the tank and out of sight.
In aquariums that do not use external canister filtration, a power head is sometimes added to the aquarium specifically to power a carbon dioxide reactor.

In-tank reactor
A variant favored by do-it-yourself aquarists involves a large
section of pipe arranged vertically, with water entering at the
top and flowing out the bottom. Because of the larger cross
section of this section of pipe, the water is slowed as it passes
through. Carbon dioxide is injected into the center of the
reactor. If the flow rate of the water is suitably adjusted, it
will match the rate of ascent of the bubbles, so that they appear
to be suspended in the water and have ample time to dissolve. I do
not have any experience with this system, but it seems to me that
the changing rate of ascent as the bubbles decrease in size works
against the system, and getting the adjustment right strikes me as
tricky.
Laboratory experiments show that aquarium plants grow fastest
with a dissolved carbon dioxide level of about 30 mg/l. This is
also close to the highest carbon dioxide concentration that most
fish tolerate without difficulty.
Now consider what takes place where carbon dioxide is injected into the tank. The carbon dioxide in the injected bubbles is close to atmospheric pressure, but it is pure carbon dioxide rather than the 0.04% carbon dioxide of room air. If you managed to saturate the tank water with pure carbon dioxide at atmospheric pressure, you'd end up with a concentration of 1.5 grams per liter, and a pH of 3.7. This would quickly kill all your fish and plants. Of course, carbon dioxide is constantly escaping from the tank, so that it would be very difficult to achieve such saturation. However, it is easy to imagine injecting enough carbon dioxide to get well above the safe limit of 30 mg/l.
There are so many variables involved in determining the balance
between carbon dioxide injected into the tank and escaping from
the tank that finding the right injection rate is a matter of
trial and error — with error initially on the side of too little
rather than too much! It requires monitoring the amount of
dissolved carbon dioxide in the tank, to ensure that it stays
around 20-30 mg/l. Electronic meters that measure carbon dioxide
in the air are readily available and not terribly expensive, but
ones designed for measuring dissolved carbon dioxide are very hard
to come by. Most aquarists use a drop bulb instead.
This is a little glass gizmo that you stick to the inside of your tank. It has a horn-shaped opening that faces downwards, trapping a big bubble of air. The opening leads to a small bulb that contains an indicator solution. The trapped air bubble equilibrates with the tank and the test solution with the air bubble, so that after half an hour or so, the amount of dissolved carbon dioxide in the indicator solution is the same as the amount in the tank.
The indicator solution is water of a known carbonate hardness with a few drops of bromothymol blue. Some vendors instruct you to simply use aquarium water, but it is better to use water with carbonate hardness close to 4 dKH. Bromothymol blue turns a vivid emerald green at a pH of 6.8, which for 4 dkH water indicates a fCO2 of about 41. This corresponds to 24 mg/l of carbon dioxide, right in the ball park.
We see here another benefit of carbonate hardness. If the carbonate hardness of the water in the tank were just 1 dkH, then the pH at 24 mg/l of carbon dioxide would be 6.1, uncomfortably low for many fish.
The chemistry of water and carbon dioxide accounts for the three
primary chemical elements of life: oxygen, carbon, and hydrogen.
In the course of the discussion, I mentioned that plants use an
enzyme called carbonic anhydrase to help them access the reserve
of bicarbonate in the water. Humans have their own version of this
enzyme, though in our case its function is to help us get rid of
carbon dioxide waste. In either species, the enzyme consists of a
strand of protein wrapped around a zinc ion.

Amino acid molecules are linked together by stripping a hydrogen off the nitrogen of one molecule and the hydrogen-oxygen group off the carbon of the second molecule, forming a water molecule, and the nitrogen linking to the carbon:
This process takes place in structures called ribosomes that use
RNA as a template for the protein. The RNA encodes the sequence of
amino acids making up the protein. The RNA in turn is copied from
DNA in the nucleus of the cell, which acts as a huge library of
recipes for all the proteins required by the cell -- tens of
thousands for a typical plant.
What does this tell us? The 21 amino acids all contain carbon,
nitrogen, hydrogen, and oxygen, so we can add nitrogen to the list
of chemical elements essential for life. The side chains of the 21
amino acids vary in complexity from a single hydrogen atom to
large carbon backbones with many other atoms attached. These
include atoms of oxygen, hydrogen, and nitrogen, but also, in some
cases, sulfur. So sulfur is a fifth chemical element essential for
life.
Ribosomes are made of protein and nucleic acid, as are RNA and DNA. Nucleic acid is itself a giant molecule, formed of various permutations of four nucleotides which all contain carbon, hydrogen, nitrogen, and sometimes oxygen. These are bonded together by phosphate groups. Our list of chemical elements essential to life now includes oxygen, carbon, hydrogen, nitrogen, phosphorus, sulfur, and zinc. There are more to come, but for now let's turn our attention to nitrogen, which is the first of the major nutrients required in addition to the primary nutrients. (The other major nutrients are phosphorus and potassium.)
Although the bulk of the nitrogen in a cell is part of a protein
molecule, nitrogen is also present in nucleic acids, in
chlorophyll and related molecules, and in many other molecules
essential to life.
Nitrogen has seven electrons, making it in some sense
intermediate in its properties between carbon and oxygen. In
particular, it has three unpaired electrons in its second shell
that can bond with unpaired electrons in hydrogen, carbon, and
oxygen. In living organisms it is almost always tightly bonded to
carbon or hydrogen, usually as part of a protein. In this state,
it is called reduced nitrogen.
Nitrogen makes up 78% of room air, so one might suppose that this
means aquarium plants have ready access to an ample supply. This
turns out not to be the case. Nitrogen in air consists of pairs of
nitrogen atoms triple bonded to each other, and the bond is
extremely strong. This makes atmospheric nitrogen chemically
inert. Some algae are able to extract nitrogen from air and break
the bond between pairs of nitrogen atoms — to reduce
nitrogen — but almost no aquarium plants are able to do so. Thus,
aquarium plants must have access to dissolved nitrogen compounds.
Proteins are constantly being broken down in an aquarium. Most
fish eat a high-protein diet, and most of the protein is used as a
source of energy rather than incorporated into the fish' own
proteins. Even algae eaters break down much of the protein in
their diet. The nitrogen from protein is almost always released
into the aquarium water as ammonia, NH3.
Ammonia is a colorless gas that one might think would rapidly
diffuse out of a fish tank. This turns out not to be the case.
Ammonia dissolves so eagerly in water that, for every milligram
per liter in aquarium water, the equilibrium concentration in the
air above the tank is just one microgram per liter. This by itself
would make for very slow diffusion of ammonia out of a tank, but,
in addition, most ammonia in a healthy tank reacts with the water
to form ammonium, which is a dissolved ion that cannot diffuse out
of the water.
Ammonia consists of a nitrogen atom with three hydrogen atoms bonded at three corners of its tetrahedral second shell. These corners have a positive charge from the presence of the protons. The fourth corner is negatively charged from its bare electron pair. This electron pair is a magnet for another proton, more so than the electron pairs of water. As a result, ammonia readily steals protons from hydronium, raising the pH of the water in which it is dissolved. Thus, it is a base. The reaction forms ammonium, NH4+:
NH3 + H2O -> NH4+ + OH-
The equilibrium between ammonia and ammonium is
[NH4+][OH-] / [NH3] = 1.78 x 10-5 M
or, using the acid-base equilibrium,
[H+][NH3] / [NH4+] = 5.62 x 10-10 M
This implies that, at normal tank pH, ammonia will almost all be
converted to ammonium.
Ammonium is a blessing and a curse. Almost all aquarium plants readily assimilate ammonium as a source of nitrogen for manufacturing proteins. Since it is already reduced, it can be attached directly to existing carbon skeletons with a modest expenditure of energy. The energy is supplied by a compound called adenosine triphosphate, ATP, which acts as a kind of universal energy currency within living cells.
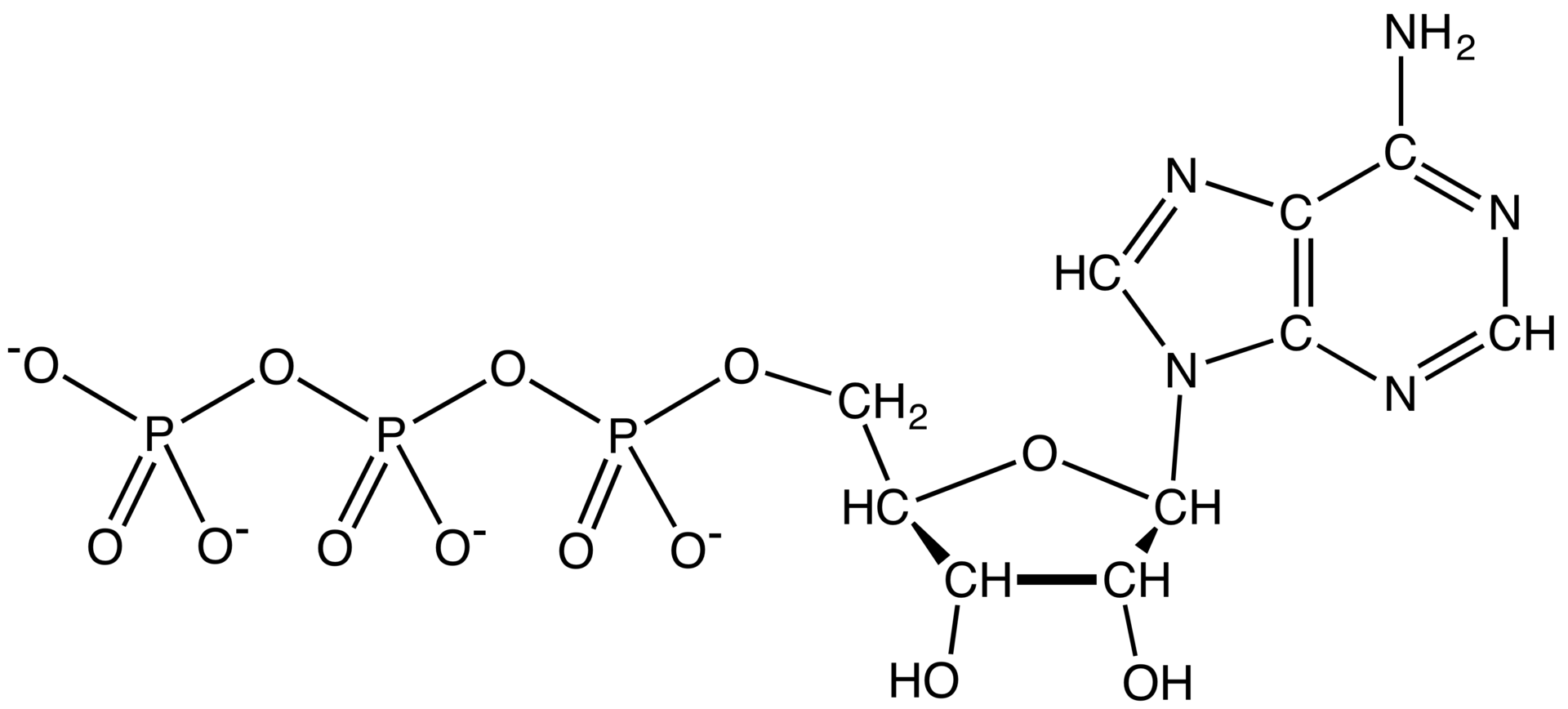
The string of three phosphate groups is the high-energy part of
the molecule. A vast number of enzymes in cells carry out
energy-consuming reactions by transferring the final phosphate
group from ATP to other molecules. This phosphorylates
the molecule while converting the ATP to adenosine diphosphate,
ADP. The phosphorylated molecule gains the energy necessary to
complete its transformation into a different molecule. In the case
of ammonia assimilation, an enzyme called glutamine synthetase
transfers a phosphate group from ATP to an amino acid called
glutamate, which "preps" the glutamate to swap the phosphate for
an ammonium ion to produce a different amino acid called
glutamine.
Glutamine synthetase consists of ten protein balls surrounding
twenty magnesium ions that act as the cutting edge of the enzyme.
We thus add another chemical element to those required by plants:
magnesium. We will find that magnesium is used in a great many
enzymes, but has perhaps its most visible role as the active
center of chlorophyll, the molecule that collects light for
photosynthesis.
So far, all the cell has done is add an amide group (that's the name for an ammonia molecule when it's stripped of a hydrogen and bonded to a larger molecule) to an existing amino acid to produce a different amino acid. But now the cell can use a second enzyme, glutamine oxoglutarate aminotransferase or GOGAT, which moves the amide from the glutamine to a molecule called α-ketoglutarate. α-ketoglutarate is a carbohydrate, containing no nitrogen; when an amide is added, it becomes a glutamate molecule. The glutamine also goes back to being a glutamate, so that glutamate acts as a catalyst in its own synthesis. The net result is that carbohydrate in the form of α-ketoglutarate is converted to the amino acid glumamate by addition of ammonium, Glutamate can then be modified by other enzymes to produce the other twenty amino acids.
GOGAT has several forms, but the form used by plants during
photosynthesis consists of a ball of protein bonded to FAD, iron,
and sulfur. FAD, flavin adenine dinucleotide, is a medium-size
molecule containing carbon, hydrogen, nitrogen, oxygen, and
phosphorus.
FAD
FAD is useful for moving electrons from molecule to molecule, an
important part of many chemical reactions (redox
reactions.) And because FAD must work with iron to make amino
acids, we can add iron to our micronutrient list.
I described ammonium as a mixed blessing. It's an ideal nutrient for supply plants with nitrogen. But ammonia (the gas form) is highly toxic to fish at concentrations as low as 0.05 mg/l, irritating their gills so that the fish cannot take in oxygen, and they suffocate. This corresponds to about 10 mg/l of ammonium (the ion form) at a pH of 7.0. At a pH of 8.5, the toxic concentration of ammonium drops to just 0.3 ppm, as the equilibrium shifts in favor of the ammonia form. Fortunately, ammonium is rapidly eliminated from an established tank by certain species of bacteria and archaea. (Archaea are primitive single-celled organisms that resemble bacteria, but have biochemistry closer to that of plants and animals.) These are slow-growing microorganisms, taking up to half a day to double their numbers even in an ideal environment. However, they are able to obtain energy for their life processes by oxidizing ammonium to nitrite:
2NH4+ + 3O2 -> 2NO2- + 4H+ + 2H2O
This requires a lot of oxygen, so the microorganisms responsible for this process tend to colonize the parts of your aquarium that have a good oxygen supply. Most aquarium filtering systems include some form of biological filter that has a large surface area exposed to both air and tank water that the microorganisms can colonize. It can take up to two weeks for the microorganisms to multiply to the point where they can bring ammonium levels below the detection limit, but thereafter ammonium should cease to be a problem in a healthy tank.
I'll say more about the specific microorganisms that consume ammonia further on.
Oxidation of ammonium to nitrate lowers the pH. The oxidation of
enough ammonium to produce 22 mg/l of nitrate is enough to reduce
the carbonate hardness by 1 dKH. Reduction of nitrate back to
ammonia by green plants reverses the process and so raises pH. On
balance, a densely planted aquarium will convert nitrate (supplied
as fertilizer) to plant tissue (remove as trimmings) and so tend
to increase in pH, while a less densely planted aquarium with many
fish will convert organic nitrogen (as fish food) to nitrate
(removed in change water) so that pH tends to decrease with time.
The latter is the more common situation, and is yet another reason
why some carbonate buffering is needed in a planted aquarium.
Ammonium levels can and should be monitored in a new tank. There
are a couple of ways this can be done. Older test kits used a
substance called Nessler's reagent, potassium tetraiodomercurate
(K2[HgI4]) dissolved in potassium hydroxide. When this is
added to a test sample, the ammonium combines with the reagent to
produce a bright yellow mixture of mercuric oxide and mercuric
amidoiodide:
NH4+ + 2 HgI42− + 4 OH− → HgO·Hg(NH2)I ↓ + 7 I− + 3 H2O
In a mature aquarium, the reagent should show no yellow color.
Otherwise, the ammonium concentration can be estimated by
comparing the depth of yellow color to a color chart.
Since mercury is highly toxic, modern test kits use Berthelot's reagent to test for ammonium. This typically uses two test solutions. One is a solution of sodium salicylate:
while the other is an alkaline solution of sodium hypochlorite — ordinary household bleach. These react with any ammonium in the water to produce indophenol, a bright blue substance:
Again, the solution can be compared with a color chart to estimate ammonium levels. This test is cheaper and much less toxic than the Nessler test, but it is somewhat less sensitive to low ammonium levels. The Berthelot test detects only ammonia, but since one or both of the reagents are normally strong alkalis, any ammonium will be converted to ammonia and then react with the reagents.
The Nessler test uses agents that are stable and nonvolatile, so
the test solution should keep for years. The Berthelot test uses
bleach, which slowly loses potency once the bottle of reagent is
opened. It should be replaced after a year or so.
The microorganisms that feed on ammonium oxidize it to nitrite. Unfortunately, this compound is also toxic to fish, with a toxic limit of just 0.3 mg/l for more sensitive species. It binds to hemoglobin in the fish's blood and blocks its ability to transport oxygen, causing much the same symptoms as ammonium poisoning.
Fortunately, a second set of microorganisms feed on nitrite, oxidizing it to nitrate:
2NO2- + O2 -> 2NO3-
These microorganisms closely resemble those that feed on ammonium, including having a slow growth rate and requiring abundant oxygen. They will be found alongside the ammonium oxidizing bacteria in a mature tank. However, since they cannot multiply until they have nitrite to feed on, they will not begin to establish themselves until after the ammonium oxidizers are established. Thus, "cycling" of a new tank takes place in two stages, with an ammonium spike followed by a nitrite spike.
Test kits for measuring nitrite use the Griess reaction. A solution of sulfanilamide (which, oddly, is also an antibiotic) is added to the sample along with an acid solution of naphthylethylenediamine. The sulfanilamide reacts with any nitrite present and with the naphthylethylenediamine to produce N-alpha-naphthyl-ethylenediamine, which is bright red in color. The color is compared to a color chart to estimate the nitrite level.
Be mindful of expiration dates on test kits, particularly tests
less frequently used. I once lost a dwarf gourami in a new
tank when I used an older nitrite test kit. The test showed no
nitrite after it had sat the recommended length of time and I
thought the tank was safe. I later set up another new tank and
found that the old test kit did not register high nitrite levels
until it had been allowed to sit much longer than the recommended
time. Most of us set up new aquariums infrequently enough that we
should simply regard fresh ammonia and nitrite test kits as part
of the cost of the new aquarium, unless we have the means to make
calibration solutions to be sure our old kits are still usable.
You may find it interesting that the Griess reaction is also sensitive to traces of high explosives and is used in criminal forensic work for this purpose. I suspect that explosive traces are not a common confounder when testing an aquarium for nitrite.
The best control is the biological one: Do not place fish in a
tank until it has built up adequate biological filtering capacity
for ammonium and nitrite. This is a bit of a chicken or egg
problem, because ammonium is needed to promote the growth of
filtering microorganisms, and fish are the usual source of
ammonium in a tank. We can solve the problem by adding an
artificial ammonium source to the tank to feed the microorganisms.
Rotting protein will work, so one can simply add fish food to the
tank, as if one was feeding the planned stock of fish, and let it
decompose in the tank. If this seems distasteful, an alternative
is to add small quantities of ammonium chloride to the tank.
Likewise, aquarists often maintain quarantine/hospital tanks for
ensuring that newly obtained fish are free of parasites before
adding them to a display tank, or for isolating sick fish from
display tanks for treatment. These tanks need biological filtering
capacity sufficient for any batch of fish likely to be put in
quarantine. This can be maintained by feeding the quarantine tank
with ammonium chloride, when empty of fish, to maintain the
biological filter.
One should begin the process of "cycling" a new tank by adding
enough ammonium chloride to bring the concentration of ammonium to
about 4 ppm. This is optimal for the filtering bacteria. This
requires about 1 teaspoon of ammonium chloride per 100 gallons of
water. When testing shows that the ammonium is beginning to be
converted to nitrite, one can begin adding additional ammonium at
a rate corresponding to the anticipated diet of the fish that will
be placed in the tank. This will keep the ammonium filtering
bacteria fed while a corresponding colony of nitrite filtering
bacteria also becomes established.
The dose rate is determined from the maximum likely feeding rate of the fish that will later be introduced into the tank. Dry fish food is typically about 50% protein, and protein is about 16% nitrogen by weight. Working through the math, and accounting for the other atoms in an ammonium chloride molecule, we find that a gram of dry fish food is equivalent in nitrogen content to about 0.63grams of ammonium chloride. This is about 0.1 cc or 1/50th of a teaspoon. Small scoops for measuring 0.15cc or 1/16 or 1/32 teaspoon are readily available from Amazon or other online suppliers. For estimating food consumption, it is helpful to know that a U.S. dime coin or a single strand of spaghetti weighs about a gram.
Ammonium is not the only nutrient required by a biological
filter, though it is by far the most important. The filter
bacteria also need phosphate and magnesium, but as little as 0.02
parts per million of phosphate is sufficient. It is likely that
all but the softest, purest water contains sufficient trace
elements to meet the requirements of biological filter bacteria.
Supplying some of the nitrogen as fish food will likely address
any deficiencies. Another approach is to regularly replace the
water in the quarantine tank with water from the display tank.
This will contain residual nutrients from the fertilizers added
for the plants in the display tank and from food supplied to the
fishes in the display tank. This approach also has the advantage
that fish in quarantine will become acclimatized to roughly the
same water conditions they will experience in the display tank.
Sometimes medication in a tank, or some other environmental
factor, will reduce the population of ammonium oxidizing
microorganisms, resulting in an ammonium spike. Symptoms are fish
gasping and floating just below the water surface. An ammonium
test can confirm this is the difficulty. The best course of action
is to change out almost all of the water in the tank to remove the
ammonium, and continue monitoring and changing out water until the
spike has passed. Because of the close relationship between
ammonium and nitrite, nitrite should also be monitored. Since it
is the ammonia in equilibrium with the ammonium that is actually
toxic, nothing should be done to the tank that might raise the pH,
such as abruptly shutting off carbon dioxide injection.
Products that claim to lock up ammonium are rarely helpful. They
do not substitute for a full water change when there is an
ammonium spike, and are actually counterproductive when cycling a
new tank, since they reduce the supply of ammonium feeding the
maturing filter. When a tank has a damaged biological filter and
there is nowhere to move its occupants, the water must continue to
be changed frequently for a prolonged period of time. Ammonium
levels that are tolerable to fish are low enough that the filter
capacity will grow only very slowly, sometimes taking many weeks
to fully recover.
Products that claim to lock up ammonium come in two varieties.
The pure liquid products claim to instantly convert ammonia to
harmless ammonium. There's only one way to do that, and that is to
lower pH, so these products cannot be anything more than mild
acids. The solid products are zeolites, which are
naturally occurring (but often nowadays synthesized) minerals with
an extremely porous structure at the molecular level. These
minerals have a very large ion exchange capacity, meaning
that the immense molecular surface area of the zeolite has binding
sites for ions that allow the zeolite to exchange a positive ion
(typically sodium) occupying these sites for the ammonium ions in
the water. I'll have more to say about ion exchange later on.
Aquarium suppliers sometimes offer bacterial cultures for rapid
cycling of a tank. While there is nothing particularly wrong with
using such cultures — I have done so myself — one should not
assume a tank is instantly cycled when such a culture is added.
Only when testing shows that no detectable ammonium or nitrite is
present, even when an ammonium source is present, should the tank
be regarded as fully cycled. The cultures merely speed the
process.
We are less certain than we would like to be on exactly which microorganisms dominate ammonia oxidation in a freshwater aquarium. Protein decomposition occurs in almost all environments and, as a result, a wide variety of ammonia oxidizers are present almost everywhere, but in relatively low numbers. The bacteria genuses Nitrosomonas and Nitrosococcus are most often mentioned, but this may simply be because they are the easiest to study. Nitrosomonas is found in natural freshwater, it enjoys conditions of pH and temperature typical of freshwater aquariums, and it's not a bad guess that one or more species will colonize your aquarium. It is a common choice for commercial cycling cultures. The bacteria use the enzymes ammonia monooxygenase (containing copper and nonheme iron), hydroxylamine oxidoreductase (containing heme iron), and an as-yet-unidentified third enzyme to do the reduction.
Nitrobacter and Nitrospira are often mentioned as
nitrite oxidizers. These are distantly related to ammonia
oxidizers, and scientists remain puzzled why only a few bacteria (Nitrobacter
may actually be one) are able to do the complete oxidation of
ammonia to nitrate, rather than just half of the process.
Regardless, Nitrobacter is also a common choice for
cycling cultures.
How effective are the cultures? It's pretty hard to say. Ammonia and nitrite oxidizers are actually pretty delicate and hard to culture as bacteria go, so there is a temptation for manufacturers to take shortcuts. If the culture does have the right species — look for the names I've mentioned on the labels — there is still the question of how well they survive through shipping and storage. Lack of oxygen, as in a sealed container, tends to cause such bacteria to go into a resting state in which they are a bit more resistant to adverse conditions, but no reliable source I've found claims they can survive more than a few months. So check expiration dates carefully. You are likely as well off to start a new tank with water and some filter medium taken from an established tank. Failing that, you can use some substrate from an existing tank. Your local fish shop may even let you have a bit of substrate from their tanks, though you will want to artificially cycle the tank for at least two weeks to ensure any parasites are starved out. If you are a beginning aquarium keeper who has no access to an established tank, you can try simply adding a pinch of garden soil to the tank. Ammonia and nitrite oxidizers are found almost everywhere, and those that can adapt to fresh water will automatically take over.
Cycling cultures do have one advantage, which is that, if they
come from a reliable source, they will be pure cultures containing
no algae spores or other pests. A "gnotobiotic" tank containing
only the precise species you deliberately introduce would be a
fascinating project, though I suspect one that would be very
difficult to maintain for long. In addition to populating its
biological filter with a pure starter culture, you'd have to use
thoroughly disinfected hardscape and only tissue-cultured aquatic
plants. Introducing fish would be a particular difficulty, since
it's not obvious how you would disinfect them.
The final product of the nitrogen cycle is nitrate. This can be used as a nitrogen source by most aquatic plants (though they prefer ammonia) and it has the considerable advantage that it is nontoxic to most fish at concentrations that are easily maintained by regular partial water changes. Because of the dangers of ammonia, most planted aquarium keepers add any supplemental nitrogen to their aquariums as nitrates rather than ammonium salts. Ideal levels are between about 10 mg/l and 20 mg/l as NO3-, though plants show a wide tolerance. The chief difficulty at high levels is that nitrate also encourages algae growth.
Plants that absorb nitrate must convert it back to ammonium to
make use of it. Conversion back to nitrite occurs within cells
using an enzyme called
nitrate reductase. This is composed of four chains of protein
wrapped around a cluster of molybdenum and sulfur atoms. That adds
another micronutrient to our list: molybdenum. The enzyme also
requires magnesium and calcium to function, which adds calcium to
our list of nutrients. Calcium is used in many other places in
plant cells, and so is required in large enough quantities to be
classified as a minor nutrient rather than a trace nutrient.
Nitrite is converted to ammonium in aquarium plants within their chloroplasts, the chlorophyll-bearing structures within plant cells that carry out photosynthesis. The enzyme responsible, nitrite reductase, contains protein and heme, a compound composed of a flat doughnut of carbon-nitrogen rings (a porphyrin) with an iron atom at the center.

Other porphyrins are common as parts of enzymes, and chlorophyll
is a magnesium porphyrin, so we'll see variations on this
structure again.
Nitrate test kits first reduce the nitrate to nitrite, then measure the nitrite level using the Griess reaction. Typically, one of the reagents is an acidic solution of sulfanilamide. The other contains the naphthylethylenediamine and the reducing agent. The reducing agent is the key. Producers of aquarium test kits invariably treat their reduction formula as proprietary, but nitrate test kits that have a powder as one of the reagents likely use nitrate reductase from Aspergillus mold cultures. These seem to be the most reliable test kits. Those that use two reagents supplied as liquids likely use a ferrous iron salt (which gives one of the reagent a yellow color) to reduce nitrate to nitrite. Ordinary bleach is also able to reduce nitrate to nitrite and may be the reducing agent in some test kits. All the mixing and shaking you are instructed to do are aimed at making sure the nitrate is fully reduced. Once nitrate is reduced to nitrite, by whatever method, the Griess reaction takes place and produces a red color that is compared to a color chart to estimate nitrate levels.
The test kit cannot distinguish nitrate from nitrite, so what is actually measured is the total of both. This is not normally a problem, since nitrite is not present in a healthy mature tank. The reducing reagent has a limited lifetime when exposed to air, since air will oxidize the reducing agent. Likewise, the hydrochloric acid used to acidify the reagents is volatile and will be gradually lost once the reagent bottles are opened. The test kit should therefore be replaced after a year, if it is not used up before them. It often will be, since the nitrate level should be tested weekly and the nitrate test tends to consume larger volumes of reagent that other test kits.
In nature, nitrate levels remain reasonably steady, because most nitrate produced as a waste product by living organisms is taken up by plants and converted back to protein. Nitrate lost by conversion back to inert nitrogen is balanced by nitrate produced by lightning strikes or ammonia produced by nitrogen-fixing algae and legumes.
However, nitrate is unlikely to remain in balance in the highly artificial environment of a fish tank. A sparsely planted tank well-stocked with fish will accumulate excessive nitrate over time. A heavily planted tank with few fish will become depleted in nitrate. The aquarist must test nitrate levels periodically and make necessary adjustments.
Excessive nitrate is remedied by periodic partial water changes.
These are a good idea on general principle, since they also remove
any buildup of organic waste or other harmful or disagreeble
substances. The aquarist can adjust according to testing; thus, if
the target is 20 mg/l, the aquarist is making 50% water changes
weekly, and the level is 30 mg/l before the water change, the
aquarist can probably get by without any nitrogen supplementation.
After the water change, the level will be diluted back to 15 mg/l
and will gradually increase during the week. If the level is
higher, more water changes may be required. If it is lower, the
aquarist may add some additional nitrate to the change water to
ensure that levels remain close to the target. Nitrate is best
added as potassium nitrate or calcium nitrate, depending on
whether potassium or calcium also need to be supplemented. I make
up a solution of 129 grams of horticultural calcium nitrate in 500
ml of deionized water (about half a pint) so that one ml (1/5
teaspoon) of this solution raises the nitrate in one gallon (four
liters) of aquarium water by 10 mg/l. "Calcium nitrate" sold for
horticultural use, with a nitrate content of 15.5% by weight, is
actually calcium ammonium nitrate, 5Ca(NO3)2•NH4NO3•10H2O, which
has a little less than 10% of its nitrogen as ammonium. This is
generally not enough ammonium to trouble fish, and it should be
quickly oxidized to nitrate in a mature aquarium.
Excess nitrate can also be removed by running water through an anaerobic filter with a carbon source. This will be colonized by denitrifying microorganisms that extract oxygen from nitrate to produce inert nitrogen. Nitrate can also be removed using electrolytic devices designed for the purpose. Neither is really needed in a planted aquarium, but electrolytic or anaerobic removal of nitrate is more common in reef tanks, where excess nitrate is a much more serious problem than in a planted aquarium.
Since nitrate should be regularly monitored, and is easily
adjusted, it is unusual to have signs of low nitrate in an
aquarium. However, if nitrate does run short, plants will show
slower growth, with leaves tending to turn yellow and the
internode distance (distance between leaves on stem plants)
becoming shorter. Older leaves will tend to die and drop off.
Unfortunately, this pattern is seen with most mobile
nutrients. Mobile nutrients, like nitrogen, are ones that are
relatively easily moved from one part of the plant to another.
Lack of a mobile nutrient shows its signs first in the older parts
of the plant, as mobile nutrients are drawn to the young, actively
growing parts of the plant. Thus nitrate deficiency can be
confused with deficiency of other mobile nutrients.
Excess nitrate is not directly harmful to plants until levels are
very high. Nor does nitrate have much tendency to interfere with
uptake of other nutrients, except phosphate at very high nitrate
levels. The chief effect of excess nitrate is to promote the
growth of algae and faster-growing green plants, which can overrun
the aquarium.
In the discussion of nitrogen, I mentioned that phosphorus is
also an essential major nutrient in a planted aquarium. Phosphate
is part of the RNA and DNA that provides the template for
proteins; it is part of the ATP that is the energy currency of the
living cell; it is part of coenzymes like FAD; and it is an
essential component of cell membranes.
Phosphorus in both the aquarium and in living organisms is present as phosphate. Phosphate is derived from phosphoric acid, whose molecules consist of a phosphorus atom bonded to four oxygen atoms, three of which are bonded to hydrogen atoms. One proton easily comes loose, with a pKa of 2.15, so phosphoric acid itself is never present in any significant quantity either in cells or in aquariums. The second proton comes loose with a pKa of 7.20, while the third comes loose at a pKa of 12.32. Thus, triply ionized phosphate is also not present in any significant quantities in aquariums or living cells. Instead, phosphate exists as a mixture of the singly and doubly ionized forms. However, it is conventional to write phosphate in chemical formulas as if it was fully ionized.
With its second pKa of 7.20, phosphate forms an almost ideal
buffer for water close to neutral pH. If hydronium is added to
neutral water containing phosphate, some of the doubly ionized
phosphate will take up most of the hydronium, which prevents the
pH from dropping very much. If hydroxide is added, some of the
singly ionized phosphate will lose a second proton to neutralize
the hydroxide, becoming doubly ionized in the process, which
prevents the pH from rising very much. The catch is that high
phosphate levels in a planted aquarium can lead to an overgrowth
of algae, and can interfere with uptake of other nutrients, so
planted aquarium keepers must avoid using phosphate-based buffers.
Phosphate is useful in cells primarily for its ability to produce phosphate esters. A phosphate ion can bind to larger molecules via one of its oxygen atoms. Such bonds are energy rich and so help drive chemical reactions. It is the high energy content of phosphate ester bonds that makes ATP the energy currency of the cell. However, in aquarium water, the high energy of phosphate ester bonds means that such bonds rarely form spontaneously, and phosphorus is almost entirely present as singly or doubly ionized phosphate.
Ideally, the phosphate level in a tank should be about one-tenth
the nitrate level. So if nitrates should be between 10 to 20 mg/l,
the phosphate level should be between 1 to 2 mg/l. Like nitrate
levels, phosphate levels should be tested regularly.
Test kits for phosphate typically consist of two solutions. The first is ammonium heptamolybdate dissolved in dilute sulfuric acid. The second is stannous chloride dissolved in glycerol. The sulfuric acid and glycerol help stabilize the reagents. When added to the test sample, any phosphate present will react with the reagents to form a complex phosphomolybdate ion that is intensely blue in color. The sample is compared with a color chart to estimate phosphate levels.
The reagents are reasonably stable and should remain usable for years. In any case, as with the nitrate test, the phosphate test should be done frequently enough that the reagents will typically be used up before they lose potency.
This is similar to control of nitrate levels. If phosphate is excessive, water changes should be increased in frequency or volume. If phosphate is deficient, supplemental phosphate can be added as potassium phosphate. I make up a solution of 7.4 grams of horticultural potassium phosphate in 500 ml of deionized water; each ml of this solution will add 2 mg/l of phosphate to 1 gallon of aquarium water.
Plants that lack phosphorus show slower growth, and if the deficiency is not corrected, they will show a characteristic reddish to purplish discoloration of older leaves. (Like nitrogen, phosphate is a mobile nutrient.) This discoloration occurs because the plants are unable to properly process sugars, which accumulate and form pigments called anthocyanins. These are the same pigments that make autumn leaves red, and are produced by the same mechanism: Autumn leaves are cold enough at night to trap excess sugar.
Excess phosphate is not directly harmful at any likely level, but
can cause considerable indirect harm by promoting algae growth and
by interfering with uptake of other nutrients. In particular,
phosphate forms insoluble compounds with many trace nutrients.
Excess phosphate is thus manifest as a seeming deficiency in trace
nutrients.
Since phosphate should be regularly monitored, both phosphate
deficiency and excess should be easy to avoid.
We haven't mentioned potassium, because it isn't directly
involved in any of the life processes we've described so far. This
is because potassium exists in nature only as the potassium ion, K+,
and the potassium ion is almost as inert as atmospheric nitrogen.
Potassium has 19 electrons. These are enough to completely fill
the first and second shell, and the lower-energy s and p orbitals
of the third shell. The final electron finds itself alone in the
fourth shell. This electron cannot get close to the nucleus
because of the inner shell electrons, and so it is bound quite
weakly to the atom. It takes very little encouragement for the
electron to leave the atom and go find somewhere closer to a
positive charge, and that electron practically never comes back.
The chemical properties of potassium arise almost entirely from
the fact that it is a large and weakly positively charged ion.
This is also where its importance to plants comes in: It provides
positive charge to counter negative charge throughout living
cells. This means that it is indirectly involved in a vast
number of biological processes. It also regulates osmotic
pressure, the tendency for water to leave a cell. Finally,
potassium helps stabilize the ribosomes that transcribe proteins
from nucleic acids, so that protein synthesis cannot take place
unless potassium is available. These functions are so important
that potassium is the third major nutrient, after nitrogen and
phosphorus.
Fish don't produce much potassium waste. As a result, in many
sparsely planted tanks, potassium is the only major nutrient that
needs to be regularly supplemented. The ideal level is about the
same as nitrate, but not less than around 10 ml/l.
Almost all potassium test kits available are aimed at reef
aquarists, who must maintain potassium at around 400 mg/l — over
ten times the recommended value for freshwater aquariums. These
tests are simply not sensitive enough to be of use to planted
aquarium keepers. However, it is sometimes possible to adapt these
kits for freshwater use, by increasing the test column depth.
Normally the color change is estimated by looking through a
small test vial with the water sample and reagents against a white
background. If the sample plus reagents is drawn into a narrower
container, so that you are looking through a greater depth, the
intensity of color is correspondingly increased. (Some tests even
give instructions on how to do this for a low range reading.)
Potassium tests calibrated for planted aquariums are just
becoming available (apparently only in Europe; they are not yet on
Amazon as of January 2020) and I have no experience with
them. This means I also have no good idea how they work.
However, some laboratory potassium tests mix the sample with
sodium tetraphenylborate, which precipitates any large, low-charge
cation to make the water turbid. The degree of turbidity is a
measure of how much of the ion is present. Potassium is the only
such ion likely to be present in a healthy aquarium. However, from
product descriptions, it appears likely that the potassium test
uses a colorimetric indicator similar to the Eriochrome Black used
to measure total hardness, which I'll describe below when I
discuss calcium.
Since kits are not widely available for testing potassium, one
can do no better than assume there is little in tap water and that
plants will not use much between water changes. Both are
reasonable assumptions. One then simply doses replacement water to
the target level. I make up a solution of 37 grams of potassium
sulfate in 500 ml of water. Each ml of this solution will add 10
mg/l of potassium to 1 gallon of water. One may also use potassium
chloride, but be aware that horticultural grade potassium
chloride, typically sold as "muriate of potassium", will have a
significant amount of red clay in it, although this seems to be
harmless in practice. It may be better still to mix potassium
chloride and potassium sulfate so that both sulfur, a minor
nutrient, and chloride, a trace nutrient, are provided to the
aquarium. One recipe is 18 grams potassium sulfate and 19 grams
potassium chloride in 500 ml water, so that 1 ml of this solution
will add 10 mg/l of potassium to one gallon of water.
Because potassium is difficult to monitor, knowing the signs of potassium deficiency in plants is valuable. Inadequate potassium produces a characteristic tendency of leaves to yellow and then die at their edges or in small patches. Particularly characteristic is the presence of pinholes in leaves. Potassium deficiency can also trap sugar in leaves, producing a discoloration that can be mistaken for that caused by phosphate deficiency. Like nitrogen and phosphate, potassium is a mobile nutrient, so older leaves are affected first.
Excess potassium is almost harmless. It does not particularly
encourage algae growth and it does not especially interfere with
other nutrient uptake, though, at extreme levels, it can begin to
interfere with trace element uptake.
Calcium is the first of the minor nutrients. (The other two are magnesium and sulfur.) It is part of a vast number of enzymes, albeit in small quantities, but also acts as a kind of electrical signal within living cells. It works together with, or sometimes opposite, potassium to regulate osmotic pressure. It also acts as a kind of "rivet" to help hold together the pectin that binds plant cells to each other.
Calcium has just one more electron than potassium, so that it has
two electrons in the fourth shell. These are easily lost, if not
as easily as potassium's single outer shell electron, and so
calcium is always found in nature as the double ion, Ca2+.
This is a smaller and more strongly charged ion than potassium,
making calcium more useful in enzymes and as an electrical
signalling ion, but it also makes calcium ion less soluble. In
particular, calcium phosphate and calcium carbonate are rather
insoluble. Tricalcium phosphate, Ca3(PO4)2,
has a solubility in pure water of 20 mg/l; dicalcium phosphate,
CaHPO4, has a solubility of 200 mg/l; and monocalcium
phosphate, Ca(H2PO4)2, has a
solubility of 20 grams/l. Note how the more acid forms are much
more soluble. What is trickier is determining from [Ca2+],
[H+], and [PO4-3-] whether there
is danger of some form of calcium phosphate precipitating out.
The solubility limits for the less soluble forms are expressed as
[Ca2+]3[PO4-3-]2 < 1.26 x 10-29 M5
[Ca2+][HPO4-2-] < 1.26 x 10-7 M2
The first of these is going to be rather sensitive to pH, since
pH controls [PO4-3-]. Indeed, the reason why
tricalcium phosphate does not precipitate at almost any reasonable
calcium and phosphate concentration is that triply ionized
phosphate is almost nonexistent at normal tank pH. We can
substitute [H+][PO4-3-] = 4.8 x
10-13 M [HPO4-2-] and
transform these to
[Ca2+]3[HPO4-2-]2 < 5.5 x 10-5 M3 [H+]2
[Ca2+][HPO4-2-] < 1.26 x 10-7 M2
At a tank pH of 7.2, where half the phosphate is in the singly ionized form and half in the doubly ionized form, we find that for a phosphate concentration of 4 mg/l = 4.2 x 10-5. that the calcium concentration can reach 240 mg/l before dicalcium phosphate precipitates and 61 mg/l before tricalcium phosphate precipitates. The solubility rapidly decreases as pH goes up.
This incidentally explains why phosphate levels in hard water may
appear low even when phosphate is generously supplemented. It is
precipitating out as tricalcium phosphate. This is a common
difficulty for farmers and gardeners, and the solution is to
supply phosphate as small pellets of the acid salts. These create
small pockets of soil with low pH in which the phosphate is
available to plant roots.
The solubility limit for calcium carbonate is
[Ca2+][CO3-2-] < 3.3 x 10-9 M2
In water in equillibrium with air, at a pH of 7.0, this means calcium carbonate does not precipitate until the calcium concentration is around 4.5 grams per liter. But as pH goes up, calcium will increasingly tend to precipitate out of solution.
If the water in your aquarium is turbid at the start of the day,
and clears as carbon dioxide is injected, there's a good chance
you are precipitating either calcium phosphate or calcium
carbonate as the pH goes up at night. This is corrected by
reducing the calcium concentration in the water.
It turns out that commercial calcium test kits are aimed mostly
at reef aquarists and are somewhat insensitive. On the other hand,
testing for calcium plus magnesium is much easier and is
the basis for freshwater commercial test kits. These are described
as measuring general hardness, often expressed in German degrees
of general hardness, dGH. One degree of general hardness is
equivalent to 10 mg/l of calcium oxide, CaO. This value is chosen
such that a degree of carbonate hardness corresponds to the same
amount of calcium as a degree of general hardness, though the two
are not necessarily equal. Adding calcium chloride, for example,
will raise general hardness while leaving carbonate hardness
unaffected.
The general hardness test is a titration test, like the test for
carbonate hardness. The number of drops of reagent added to a
measured sample of water before the reagent changes color is equal
to the general harness in German degrees. The reagent contains
EDTA plus a less powerful chelating agent called Eriochrome Black
T. In alkaline solution, in the absence of calcium, Eriochrome
Black T is intense blue in color. When free calcium is present,
the dye chelates the calcium, changing color to intense red.
When a drop of general hardness testing reagent is added to a sample, the EDTA in the sample binds to calcium and magnesium. Both form a colorless complex with the EDTA. Any calcium left over binds less tightly to the Eriochrome Black T, producing the red color. As more reagent drops are added, a point is reached in which all the calcium and magnesium are bound to EDTA, leaving none to bind to the Eriochrome Black T, which then turns blue. Many test kits use a yellowish counter dye to change the colors to orange and green, which are easier for some aquarists to distinguish at low concentrations.
The general hardness test is quite reliable, so long as the pH is
not too far from neutral, but it cannot distinguish calcium from
magnesium or other divalent metal ions and it is inaccurate for
very low general hardness. Be aware that it can take a few seconds
for the reagent to fully react with the sample, so the sample
should be thoroughly shaken and allowed to sit for a few seconds
to see any color change before adding another drop of reagent.
Calcium levels are not often a problem so long as there is enough general hardness in an aquarium and so long as levels are not so high that phosphate becomes unavailable. A general hardness anywhere between 5 and 10 is reasonable for most fish and plants, though some soft water species such as discus will be happier with lower values. If it is necessary to raise calcium, this can be done by adding calcium chloride. I make up a solution of 56 grams anhydrous calcium chloride in 500 ml of water. Each milliliter of this solution will add 10 mg/l of calcium to a gallon of water, equivalent to about two German degrees of hardness. Some adjustment may be required for commercial calcium chloride, which often contains significant moisture.
If the water supply is too hard, the hardness can be reduced by diluting with deionized water. This may require investing in a reverse osmosis system to be economical over the long term. While carbonate hardness can be reduced chemically at low cost, as discussed previously, general hardness cannot.
Unlike the major nutrients, calcium is an immobile nutrient; once
fixed in plant cells, it is not easily relocated to other parts of
the plant. Like potassium deficiency, calcium deficiency causes
leaves to begin dying, but it is the newest growth that dies
first.
Excess calcium is not directly toxic, but it renders phosphate
insoluble, particularly at higher pH. Thus excess calcium can
cause phosphate measurements to be stubbornly low in spite of
supplementation, and phosphate deficiency can follow. This can be
corrected either by softening the water or by lowering the pH. In
tanks with injected carbon dioxide, the daily decrease in pH will
redissolve precipitated calcium phosphate and make it available to
plants, so the high pH during dark periods is not the problem one
might anticipate.
Magnesium is the second of the minor nutrients. It is part of
chlorophyll and many enzymes, albeit in small quantities, and,
like calcium, it acts as a signaling ion.
A magnesium atom has twelve electrons. Two fill the first shell and eight the second, so, like calcium, magnesium has two outermost electrons that are easily lost, and it always exists in nature as Mg2+. Chemically, its chief distinction from calcium is that it is a smaller, lighter ion.
As with calcium, magnesium phosphate and carbonate are somewhat
insoluble.
[Mg2+]3[PO4-3-]2 < 1.04 x 10-24 M5
[Mg2+][CO3-2-] < 1.58 x 10-8 M2
However, the amount of magnesium in a healthy aquarium is
sufficiently less than calcium, and the solubility sufficiently
greater, that it is rare for magnesium to precipitate out of
solution.
Magnesium test kits are aimed at reef aquarium keepers and lack sensitivity. If general hardness is adequate, one must hope that a sufficient fraction of it is due to magnesium. The ideal ratio is about 3 to 4 parts calcium to 1 part magnesium, which is close to the value in most water sources. If the aquarium keeper suspects magnesium is deficient, he may add it as magnesium sulfate, Epsom salts. I make up a mixture of 205 grams Epsom salt in 500 ml of deionized water. Each milliliter of this solution will add 10 mg/l of magnesium to a gallon of water. Note that this is very nearly a saturated solution, so it takes some effort to get all the Epsom salts to dissolve.
Because magnesium is difficult to directly monitor, aquarium
keepers should be aware of the signs of magnesium deficiency.
Magnesium is a mobile nutrient, so older leaves are affected
first.The leaves become yellow, particularly between their veins,
and then begin to die (a condition called chlorosis.)
Excess magnesium can interfere with calcium absorption (and vice
versa) and so will produce signs of calcium deficiency. However,
excess magnesium is a rare occurrence.
The third minor nutrient is sulfur. This is a part of almost all
proteins and also combines with iron or other heavy metals to form
the reaction site in some enzymes. In proteins, it often serves to
link protein strands together.
Sulfur in a healthy aquarium is almost always in the form of sulfate, SO42-. This can absorb protons, but only feebly, so bisulfate (HSO4-) has a pKa of 1.99 and is almost nonexistent at normal pH. Sulfate, like nitrate, must be reduced before it can be used in protein by green plants. The enzymes involved are not as well understood as with nitrate, but we know that sulfate is first substituted for the final phosphate of an ATP molecule to produce diphosphate and adenosine phosphosulfate (APS). This is done by ATP sulfurylase in chloroplasts. APS reductase then reduces this to sulfite using glutathione, which in turn is reduced using NADPH from photosynthesis. The sulfite is quickly reduced to sulfide by sufite reductase using more NADPH, Suflite reductase contains an iron-sulfur cluster to help carry out the reaction, The sulfide is then used by cysteine synthase to manufacture the sulfur-bearing amino acid, cysteine, from serine and acetate.
Sulfide from breakdown of proteins in an aquarium takes the form
of hydrogen sulfide. Hydrogen sulfide is extremely toxic,
but fortunately it reacts almost immediately with nitrate and more
slowly with room air and is converted to harmless sulfate. Some
bacteria are also able to oxidize sulfide to sulfate. As a result,
sulfide toxicity is rare in planted aquariums. However, in
portions of the aquarium where oxygen is deficient (such as deep
in the substrate or under large aquarium decorations) it can
linger long enough to combine with iron or other metals as
insoluble black metallic sulfides. These are mostly harmless, but
can cause unsightly black staining.
It is neither normally necessary nor easy to test sulfate levels in an aquarium. Sulfate is present in small amounts in most water supplies and is also present in fish waste, usually in sufficient quantities for the modest needs of aquarium plants. If there is any suspicion that sulfate levels are insufficient, sulfate may be added in combination with other nutrients, as potassium sulfate or magnesium sulfate.
If there is strong reason to believe sulfate is a problem, there are test kits that measure sulfate by adding barium nitrate to a water sample. Barium sulfate is extremely insoluble, so adding barium nitrate to sulfate-bearing water creates a colloidal white precipitate whose opacity is a measure of sulfate levels. This test is also sensitive to sulfite, but can be thrown off at high pH by carbonate.
Imbalance is rare, but if sulfate is lacking, young leaves will tend to turn uniformly yellow without newest growth dying off. Sulfur is an immobile nutrient, so older leaves are less affected.
Excess sulfate is almost entirely harmless, and also uncommon
with sensible fertilization.
Iron is the first of the trace nutrients, required only in minute
quantities, but it is the trace nutrient needed in the largest
amounts and most likely to be deficient in an aquarium. It is a
key component of a great many enzymes and of proteins that store
or transfer electrons. Some of these are part of the
photosynthetic structures in cells, so photosynthesis cannot take
place without iron.
An iron atom has 26 electrons. Two fill the first shell, eight fill the second shell, and another eight fill the inner part of the third shell. Six electrons fill the outer part of the third shell and two are left in the fourth shell. Those in the fourth shell are relatively easily stolen away by other electron-hungry atoms, forming ferrous iron, Fe2+. But there are five d orbitals in the outer part of the third shell filled by six elecrons. Four of these orbitals contain a single electron while the fifth has two electrons. It turns out the second electron in this pair is also relatively easily lost, producing ferric iron, Fe3+. The ability of iron to flip between these two ionizations explains much of its usefulness for moving and storing electrons in enzymes.
Unfortunately, it also accounts for the frequent deficiency of iron in aquariums. A ferrous ion has almost the same radius and charge as a magnesium ion, and is moderately soluble if the pH is not high. At high pH, it precipitates as either carbonate:
[Fe2+][CO3-2-] < 3.13 x 10-11 M2
or as the hydroxide:
[Fe2+][OH-]2 < 4.87 x 10-17 M2
At neutral pH, the maximum ferrous iron concentration is nearly
270 mg/l. Such iron levels would be so high that they would poison
everything in the tank. Thus solubility of ferrous iron is not a
serious limitation on iron availability to plants.
The problem is that, in water exposed to air, the ferrous iron oxidizes to ferric iron:
2Fe2+ + 2H2O + O2 -> 2Fe3+ + 4OH-
There is virtually no tendency for the reverse reaction to take place at normal aquarium oxygen levels, so while the reaction is not instantaneous, it steadily converts ferrous iron to ferric iron. One study found that
T1/2 = 292.899 - 0.3278 x 1014(OH- ) 2 - 182.931 log(dKH)
where T1/2 is the half-life in minutes of ferrous iron
in oxygenated water. At pH of 7.0 and dKH of 4, this means that
half a dose of ferrous iron salts will be oxidized to the ferric
form in three hours.
And ferric iron is very insoluble:
[Fe3+][OH-]3 < 2.79 x 10-39 M2
At neutral pH, the maximum ferric iron concentration is
effectively zero. It does not reach even 0.1 mg/l until the pH
drops to 3.93, far below what aquarium inhabitants can tolerate.
It is thought that plants in a natural environment obtain their
iron by secreting small amounts of citric acid or other organic
acids around their roots, Citrate is a chelating agent, like EDTA,
though less powerful. It is able to pull iron into solution and
deliver it to the plant roots in adequate quantities. It is also
likely that the low oxygen levels around plant roots allows some
ferrous iron to remain unoxidized to ferric iron.
Iron can be measured using various colored chelating agents, of which bathophenanthroline is a popular choice. This is provided as a powdered mixture with sodium bisulfite, which acts both to reduce any ferric iron in the test sample and to acidify the sample enough to dissolve the bathophenanthroline.

Bathophenanthroline is a rather expensive reagent needed in small amounts, so mixing it with a bulkier powder also simplifies dosing. The powder is mixed with a few drops of the test sample in a shallow container, often a plastic bubble dish, and allowed to react. When iron is present, the bathophenanthroline binds with it to produce an intense magenta color that can be compared with a color chart to estimate iron levels.
Unfortunately, bathophenanthroline iron tests are unable to detect iron in many of the forms in which it is added to a tank by aquarium keepers. Whereas ferrous salts register within a few minutes, iron gluconate may not fully react for up to an hour. Fe EDTA and Fe DTPA do not react with bathophenanthroline at all, so the test is useless for measuring the level of the more tightly chelated forms of iron. It is also likely to register colloidal ferric iron that is not actually available to plants.
This almost always means supplementing iron, since it is rare for
iron to be in excess and quite common for it to be deficient.
Normal water supplies will show no indication of iron with the
bathophenanthroline test. Iron can be added as ferrous salts,
which are quickly available to plants but as quickly oxidize into
uselessness, or as iron chelates. Iron glauconate and iron citrate
are readily metabolized by plants but bind weakly enough that the
iron will oxidize relatively quickly. Iron EDTA has a longer
useful lifetime in water but is more slowly absorbed, while iron
DTPA has a quite long lifetime but is poorly absorbed. And if
terms like "quite long" seem uselessly vague, this is another
example of aquarium keeping as art. The half-life depends not only
on the form in which the iron is introduced, but the pH and
hardness of the tank water, how well it is oxygenated, how quickly
plants assimilate the iron, and even on what microbial community
is present in the tank. (Bacteria catalyze a lot of reactions.)
Dedicated aquarists sometimes use a mixture of chelates to
provide a spectrum of availability and lifetime. My own practice
is to use CSM+B, which contains EDTA, iron, and most other trace
elements required by aquatic plants as a single mixture. I mix 3
1/8 teaspoons (15.6 ml of the dry powder) into 500 ml of deionized
water. One ml of this solution will raise the iron concentration
in a gallon of water by 0.5 mg/l. The recommended concentration in
tank water is between 0.1 and 0.5 mg/l.
It is very common for aquarium keepers to spread out their dosing of iron and other trace elements as much as possible, adding small quantities daily rather than a large dose weekly, for example. This seems to work well. I once had a tank in which many of the plants were languishing, in spite of what seemed like ample fertilization, but when I switched from one big dose of trace elements weekly to small doses every day, the improvement was spectacular. Some fertilization schemes call for trace elements to be added on alternating days with major nutrients. This is largely to avoid reactions with phosphate that produce insoluble metal phosphate compounds.
Older aquarium keepers sometimes used laterite to supply
iron. Laterite is a type of soil common in the tropics that
consists of a mixture of iron and aluminum hydroxides and has a
deep red color. It also contains some clay minerals, though it is
not correct to describe it as clay. It is the usual soil in
tropical stream beds and so it seemed reasonable to assume that
tropical aquarium plants could obtain iron from it. It was added
to the tank substrate as an admixture, and it may indeed promote
growth of some aquarium plants if it is fine grained
enough to create an oxygen-poor environment the plants are adapted
to. Most modern aquarium keepers rely on chemical fertilization
instead.
Iron is an immobile nutrient, and iron deficiency manifests as a characteristic yellowing of new growth (chlorosis), which may be so light as to be almost glassy. Veins are typically darker green. Chlorosis due to iron deficiency can be distinguished from chlorosis due to magnesium deficiency in that it is the new growth, not the old, that is most seriously affected.
Iron toxicity is unusual in a well-aerated tank sensibly
fertilized. Reddish staining is a sign that there is much more
iron in the tank than the plants can use. Some algae, particularly
staghorn algae, proliferate when high iron levels are present.
All other trace elements are required in smaller quantities even
than iron. They are almost never tested independently in the home
and are usually dosed along with iron in a trace nutrient mixture
such as CSM+B. The assumption is that if the iron concentration is
adequate, the other minor elements will be adequately supplied as
well. This is a reasonable assumption for most aquarium keepers.
In any case, the only practical way to check other trace elements
is to send a sample of water to a commercial testing laboratory
for analysis. This is not too expensive (I have seen quotes of $30
per sample) and may be worthwhile if plant growth is poor and the
reasons are unclear.
For every 100 milligrams of iron, CSM+B supplies 28 mg of manganese, 12 mg of boron, 5.7 mg of zinc, 1.4 mg of copper, and 0.86 mg of molybdenum. Oddly, it also supplies 21 mg of magnesium, too small a quantity to be very useful. The boron will be present as borate ion and the molybdenum as molybdate, while the other trace nutrients will all be EDTA chelates.
Manganese is part of several important enzymes, including the enzyme that actually splits water during photosynthesis. It also detoxifies oxidation products in both plants and animals. The Mn2+ ion behaves very similarly to iron, but is the most stable state at normal oxygen levels and so does not suffer from oxidation. However, its solubility product is
[Mn2+][OH-]2 = 2.2 x 10-16 M3
so the solubility varies strongly with pH. At neutral pH, the
maximum concentration of Mn2+ is 1.2 grams per liter;
but at pH 8.5 it is just 1.2 ppm. However, this should be more
than adequate.
Manganese carbonate is also low in solubility, with a solubility product
[Mn2+][CO3-2] = 8.8 x 10-11 M2
For neutral water in equilibrium with air, the maximum
concentration of Mn2+ is 170 ppm. However, at optimal
CO2 injection of 30 mg/l, this drops to 3.4 ppm. Fortunately,
aquarium water is usually on the acid side when carbon dioxide is
being injected, raising the solubility.
The more serious problem is the extremely low solubility of
manganese phosphate. I cannot find a solubility product tabulated
anywhere online, suggesting it is very low indeed. The solution
(heh) to this problem, as with iron, is to chelate the manganese.
Oddly, manganese is better absorbed by plants with an ample
phosphate supply.
Manganese is an immobile element whose deficiency produces
symptoms similar to those of iron deficiency. However, it produces
a less even yellow discoloration and veins stand out less clearly.
The distinction is not terribly important, since the corrective
steps (make sure pH is not too high, avoid excessive phosphate
levels, and fertilize with small daily doses of CSM+B) are the
same.
Boron has been known to be essential to plant growth for almost a century, but it is only in the last couple of decades that its role has begun to be really understood. It is essential for moving sugars through cells walls. Sugars have a lot of exposed protons on the surfaces of their molecules, and this makes it difficult for them to pass through the hydrocarbon layer of cell membranes. The sugar molecule is strongly attracted to water instead. Borate ions help temporarily neutralize the surface charges to allow the sugars to pass. Boron also seems to play an essential role in the formation of cell walls.
The ideal ratio of boron to calcium is about 1 to 300. Thus,
water with a general hardness of 8 should have around 0.14 mg/l of
boron. Excess boron is toxic to citrus fruit, but may be
less of a concern with normal aquarium species.
Boron is soluble in almost all its compounds and so borate can be added straight to the tank in the tiny quantities required.
Boron deficiency is surprisingly common in terrestrial crops and
can be assumed to be a frequent problem in unfertilized aquariums.
It is another immobile nutrient, whose characteristic signs are
destruction of new growth buds. These become chlorotic, brittle,
and twisted. It can be difficult to distinguish from calcium
deficiency, but the latter is much less common.
Zinc is found in some enzymes, including some of those responsible for DNA transcription. It almost always is found in the +2 oxidation state, and, like manganese, its hydroxide and carbonate are relatively insoluble. Like iron and manganese, it is best supplied in chelated form.
Zinc deficiency is marked by yellowing of middle leaves, rather than oldest or youngest. It is otherwise similar to iron or manganese deficiency. Like boron deficiency, it is surprisingly widespread in terrestrial crops.
Excess zinc is fairly toxic to plants. Its symptoms are yellowing
and reddening of younger leaves, followed by dead patches. Some of
these symptoms may arise from zinc interfering with uptake of
phosphorus and iron. Recommended levels are between 2% and 20% of
the iron level.
Copper is a part of the electron transport system in
photosynthesis and respiration, and plays a role in some other
enzymes. Its +1 state is fairly soluble but the +2 state is quite
insoluble, so, like other metals, it is best supplied as a chelate
if needed. Only very small amounts are required, and larger copper
concentrations are toxic to invertebrates, some fish, and plants.
For this reason, copper tests are available, but I have not used
them and do not have a good feel for how they work.
It is another immobile nutrient that causes young leaves to
yellow and their tips to die back. It is also toxic at excessive
levels, which vary greatly from plant species to species.
Molybdenum is required for nitrate metabolism. Requirements are miniscule but deficiency is still possible for this relatively rare element. It is supplied as molybdate, which has more than adequate solubility under most normal aquarium conditions.
It is a mobile nutrient, whose deficiency mostly affect older leaves. These yellow, curl, and die back in spots.
Chlorine has seventeen electrons, which is just one electron shy
of enough to fill its inner two shells and the inner part of the
third shell. It is hungry for an additional electron to fill the
gap, and so exists in a healthy aquarium exclusively as the
chloride ion, Cl-. Chloride is a general source of
negative charge in living organisms. It is a normal constituent of
almost all natural water supplies and is easily supplemented as
part of other fertilizers, such as potassium chloride or calcium
chloride. Chloride deficiency is characterized by a pattern of
yellow and dying spots on older leaves that have a very sharp
boundary between healthy and diseased tissue. Chlorine is not
required by ferns or mosses and can interfere with nitrate
absorption if levels are excessive.
Nickel is needed in minute quantities for nitrogen metabolism. It's a wonder of modern science that we are able to produce hydroponics solutions so chemically pure that we can prove that nickel is necessary. Nickel is a common contaminant of iron, with some iron ores containing up to 2% of nickel, so nickel deficiency is very unlikely to be seen in a planted aquarium.
Other trace elements are known to be required for the growth of
at least some plant species, but have never been observed to be in
short supply compared with the tiny amounts required. Sodium is
required by so-called C4 plants, which constitute about 3% of
plant species. The only one likely to be found in an aquarium is
dwarf hairgrass, and sodium is present in adequate quantities in
all but the softest water.
Other trace elements are needed only by a few species unlikely to be found in aquariums, or are beneficial but not strictly essential.
In unplanted aquariums, it is considered good practice to use a three-component filter. This consists of some kind of semi-permanent foam or biowheel medium that is colonized by nitrifying bacteria to form the biological filter, and a replaceable floss filter containing activated carbon that provides mechanical and chemical filtering.
The biological filter is important for rendering ammonium waste
harmless, and the mechanical filter helps remove debris from the
water. The chemical filter consists of a substance that adsorbs
impurities in the water. This is usually activated carbon, which
is amorphous carbon (such as charcoal or coked coal) that has been
treated to vastly increase its porosity and thus its surface area.
Traditionally, this was done by steam treatment, but many other
activation processes are now used.
The surface area of activated carbon is enormous in relation to
its volume, and the surface is chemically active, tending to form
various kinds of weak chemical bonds with molecules it comes in
contact with. When fluid (water or air) containing impurities is
passed through activated carbon, the impurities will tend to
weakly bond to the carbon (adsorption) and be removed from
the fluid.
Not all materials are removed equally well. When the fluid is water, as in an aquarium filter, the tendency is for ions to attract water molecules, which crowd around the ion. Positive ions will attract the corners of the water molecules that do not have protons, while negative ions will attract the protons. The crowd of water around an ion is called a solvation shell and it helps hold the ion in solution. For smaller ions, the solvation shell interferes with weak bonding to activated carbon, and so mineral salts providing the major and minor nutrients in a planted aquarium are relatively unaffected by activated carbon filtering.
This is not true of trace nutrients provided as chelates. The chelated ion is a relatively large group of atoms surrounded by a relatively weak solvation shell, and activated carbon can bind chelates. This accounts for the phenomenon of a tank that is being supplied with chelated trace nutrients nevertheless showing signs of nutrient deficiency.
Unfortunately, although I have experimented with removing carbon from my filter system, I cannot recommend the practice. Carbon filtration covers a multitude of sins. It provides considerable protection from toxins that find their way into the tank, whether from the air in the room, from an unwashed hand carelessly placed in the tank, or even from toxins generated in small amounts by cyanobacteria or other uninvited tank inhabitants. The best practice, in my judgement, is to use carbon filtering but fertilize frequently with trace nutrients to make up for those absorbed by the carbon..
Ideally, we would like to use tap water in our aquariums. It's readily available and inexpensive. However, we worry about the water quality, and with good reason: What is tolerable for humans drinking modest quantities of water may not be tolerable for fish and plants constantly submerged in the stuff.
Waterborne diseases like hepatitis, cholera, and typhoid were
once a scourge. Nowadays they are practically unknown in the West,
due to effective public health measures. One of the most
cost-effective of these is chlorination of water supplies. My
utility reports that it chlorinates my water supply to 1 ppm
chlorine equivalent, appropriate for a utility drawing its water
from a clean aquifer. Many other utilities use chloramine, which
is less likely to react with other substances in water to produce
harmful byproducts and which remains active in the water for much
longer.
Levels of chlorination that are harmless to humans are toxic to fish, which absorb water directly into their bloodstreams. The chlorine or chloramine present in almost all tap water must be neutralized before the water is safe to use in aquariums. Chlorine can be removed by letting the water sit in a bucket with an air stone or other source of aeration for a few days. The air bubbling through the water flushes out the chlorine. Chloramine takes considerably longer to be removed this way and, because your utility can switch from chlorine to chloramine without notifying you, you have to assume the worst. Some chloramine can be removed by the carbon (activated charcoal) filtration installed in most aquariums, but it is best removed before it even goes into the tank by reacting it with a mild reducing agent.
Chlorine and chloramine, like oxygen, are hungry for electrons. This makes them oxidizing agents, and their ability to strip electrons from other molecules is what makes them effective at destroying dangerous microorganisms. They are neutralized by providing a fast and easy source of electrons, which is called a reducing agent. The reducing agent must react readily with chlorine and chloramine to produce harmless reaction products, and it must not itself be highly toxic. The usual choice is a sulfur-containing compound such as potassium metabisulfite:
In other words, chlorine reacts with metabisulfite and water to produce sulfate, chloride, and hydronium. During the reaction, the chlorine pulls electrons from the sulfur and water. The reaction of chloramine with metabisulfite is similar, but produces ammonium as well. The small amount of hydronium produced is not enough to disturb the pH, and chloride, sulfate, and ammonium are all plant nutrients. None are harmful to fish except ammonium, and the ammonium is quickly removed from a healthy, mature tank by biological filtration. The reaction is almost instantaneous, and since metabisulfite is fairly nontoxic (it is even used as a food additive), it is better to add more than is needed than less. The excess will do no harm and will gradually oxidize to sulfate.
Nitrate, like chlorine, is an oxidizing agent, which raises the
question whether nitrate in aquarium water can reduce the
effectiveness of dechlorinators. I have seen no evidence that this
is the case. Chlorine is the more powerful oxidizing agent (it can
even oxidize nitrite to nitrate), and nitrate likely cannot
compete with chlorine to react with dechlorinators. However,
nitrate may help speed up the oxidation of excess dechlorinator,
being itself reduced to nitrite that is then promptly reoxidized
to nitrate by the biological filter.
If you know how much chlorine equivalent your utility puts in
your water, you can calculate how much dechlorinator to add. EPA
regulations permit up to 4 ppm of chlorine in drinking water to
ensure destruction of pathogenic organisms. My utility puts in 1
ppm chlorine equivalent, which is a more typical value where the
water source is known to be fairly clean. This is equal to
0.014 moles per liter of Cl2, so, from the reaction, I
see that I need to add 0.007 moles per liter of potassium
metabisulfite, which comes out to 1.6 ppm. I make a stock solution
of 3.2 grams dissolved in 500 ml of distilled water, and then I
simply add 1 ml of the stock solution to each gallon of my tap
water to be dechlorinated.
Aquarium keepers rarely test chlorine levels. If in doubt, you
should assume the regulatory limit of 4 ppm and dose with
dechlorinator accordingly. If you are still curious about your
chlorine levels, you can purchase a test kit for chlorine designed
for restaurants, brewers, and swimming pools. These tend to be
somewhat low in sensitivity, being designed to detect chlorine
levels of up to 100 ppm, but some will detect chlorine down to 0.5
ppm. Most work by using DPD (N,N-Diethylparaphenylenediamine) as
an indicator. This reacts instantly with free chlorine to produce
a red color that can be compared to a color chart. Chloramine can
be detected by adding potassium iodide to the test sample plus
indicator.
Persons with a normal sense of smell can detect chlorination at
the part per million level in tap water. If your tank water has
any hint of chlorine smell after you have done a water change and
allowed time for the water to react with your dechlorinator, you
are not using enough dechlorinator.
Tap water occasionally contains traces of heavy metals, particularly in houses with older plumbing. Prior to 1980, copper water pipes were often soldered with lead-based solder. Copper is likely to remain in common use, as it poses no toxic threat to humans so long as the water is slightly alkaline. However, invertebrates are much more sensitive to copper than people, and many water conditioner formulations contain EDTA to neutralize heavy metals. Since EDTA complexes are generally soluble, the heavy metals remain in solution, but in a form that is poorly absorbed and broken down by fish. In addition, the chelated metal is likely removed from water by activated carbon filters.
A peculiarity of my water supply is a high content of dissolved
silica, as much as 100 ppm according to some local water reports.
This is due to a quirk in our local geology, where the aquifer is
rich in volcanic ash. However, our water supply is not unique in
this regard, and other aquarium keepers may also suffer from high
silica levels.
Excess silica in our water supply has two consequences for
aquarium keeping. The first is that evaporation produces a "lime"
coating that is actually silica and cannot be removed by the usual
acidic lime removal chemicals. Only a strong base, such as oven
cleaner, has much effect on it. Unfortunately, anything that
effectively removes silica residues is a hazard for etching
ordinary glass, which is mostly silica.
The second consequence is that excess silica encourages the growth of diatoms. Fortunately, this can be controlled by other means, as will be covered in a later section.
Silica is normally removed from water supplies by adding alkali to the water. Unfortunately, this raises the carbonate hardness, which may be undesirable if this is already high. Silica can also be removed by ion exchange or reverse osmosis. In my case, and I suspect in most cases, I've simply learned to live with silica-rich water.
There is nothing particularly wrong with using a commercial
aquarium water conditioner. In addition to a dechlorinator and
EDTA, these may contain some inorganic salts to reduce osmotic
stress, and they typically contain "slime coat" advertised as
helpful for reducing stress on fish. I make my own dechlorinator
because I'm already adding EDTA to the tank as part of
fertilization, I'm already monitoring electrolytes, and I am
skeptical of the "slime coat." Or, really, just because I
like knowing exactly what's going into my tank. You needn't be as
fanatical.
But I feel like I should say something about "slime coat".
Fish secrete a coating of mucus that covers their entire bodies. This is not significantly different from the mucus in our own respiratory and digestive tracts. It's composed mostly of glycoproteins, which are water-soluble protein chains to which simple sugars have been bonded. These form a colloidal mixture with water that is viscous, slippery, chemically quite stable, and well-buffered. Because it is fairly impermeable to ions, it helps reduce osmotic stress on the fish. The mucus also contains inorganic ions, antibodies, and antibacterial enzymes such as lysozyme. These allow mucus to protect underlying cells and to help ward off microbial intruders and parasites. The slime coat on a fish serves as its outermost defense, like the epidermis of our own skins.
The "slime coat" in commercial water conditioners is
proprietary, but typically is either polyvinyl alcohol,
polyvinylpyrrolidone, or aloe vera extract.
Polyvinyl alcohol is an inexpensive, water-soluble, nontoxic resin that duplicates some of the mechanical properties of mucus. It is harmless enough that it has FDA approval for use in such things as eye drops. It will almost certainly do your fish no harm; the question really is whether it does them any good. It cannot duplicate the chemical and biological properties of mucus, and I've seen no evidence it actually adheres to fish skin, let alone replaces or supplements natural mucus in any way.
Polyvinylpyrrolidone is similar to polyvinyl alcohol and has even more approved uses in medicine. Like polyvinyl alcohol, it appears to be perfectly safe for fish, and the skepticism is over whether it actually confers any benefits.
Aloe vera is a natural extract of a succulent plant originally
from the Arabian peninsula. It has a long history of use in herbal
medicine, and is now widely used in over-the-counter burn
ointments and other medications. However, "natural" is not the
same as "safe" — a lesson that almost cannot be driven home too
much — and scientific research on it is mixed. We know
two things with any confidence: It is probably harmless as a
topical ointment for humans, and it is probably carcinogenic when
fed to rats in its raw form.
The bottom line? Aloe vera is not something I am comfortable
putting in my aquarium. Polyvinyl alcohol and polyvinylpyrrolidone
are almost certain safe for your aquarium, but probably don't
actually do any good. I see no harm in using a commercial water
conditioner containing the latter two, but I'd avoid aloe vera,
and I'd put no faith in the "slime coat" component of any water
conditioner.
Assuming the water supply is dechlorinated and free of toxic levels of heavy metals, the remaining considerations are its carbonate and general hardness and osmotic potential. I've already discussed the chemistry of carbonate and general hardness. The aquarium keeper should test these parameters using samples of his water supply that have been given ample time to equilibrate with room air. To these parameters, we add osmotic potential, usually expressed in terms of total dissolved solids.
Osmosis refers to the tendency of water to flow from regions with little dissolved solids to regions with more dissolved solids. This actually produces a measurable pressure, called the osmotic potential, with the numerical value
= cRT
where c is the concentration of dissolved solids in moles per
liter, R is the ideal gas constant, and T is the absolute
temperature (75 F = 298K). This tells us a solution with a
concentration of 1 mole per liter will absorb pure water from
nearby sources at room temperature against a pressure difference
as high as 25 atmospheres. There are some wrinkles to keep in
mind, among them that the concentration is of total individual
dissolved ions and molecules. Adding a mole of table salt (58.44
grams) to a liter of water actually produces a concentration close
to 2 moles per liter so far as osmosis is concerned, because each
mole of table salt breaks up into a mole of sodium ions plus a
mole of chloride ions.
Osmotic potential is a challenge for any organism living in fresh
water. Living cells contain a fair amount of dissolved solids,
and, in the absence of mechanisms to fight osmotic potential, they
would absorb water until they swelled up and popped like a
balloon. In fact, this happens to human blood cells exposed to
fresh water, which is why intravenous fluids given to patients in
a hospital must be isotonic, that is, must have enough
dissolved solids to match the solids dissolved in the bloodstream.
Some organisms, such as bacteria or plant cells, build a strong cell
wall around their cells that can resist high osmotic
potential. Protozoa use a different strategy, having small
organelles called contractile vacuoles that constantly
pump water back out of the cell as fast as it diffuses in.
Animals maintain a comfortable osmotic potential in their blood, but freshwater aquatic animals, such as fish, are in contact with nearly pure water and must constantly rid their systems of excess water that diffuses into their bloodstreams. This becomes very difficult when the water is extremely low in dissolved solids, which is one reason why most fish require at least a small amount of general hardness in their water. We sometimes help reduce stress on sick or injured fish by putting them in a hospital aquarium with added salt in the water, to reduce osmotic stress.
Osmotic potential can be measured directly by measuring the pressure that builds up in a pure water sensor with a semipermeable membrane placed in the aquarium. However, the potentials in a freshwater tank are low enough that sensors sensitive enough to directly measure it are costly. Instead, aquarium keepers tend to use two proxies for osmotic pressure. One is total dissolved solids; the other is conductivity.
Total dissolved solids is simply the weight, in parts per million, of solids left behind when a sample of water is evaporated. If a liter of water is boiled down, and the resulting solid residue weighs 200 mg, then the TDS is 200 mg/l. This is a difficult measurement to make, requiring a sensitive laboratory balance, and it does not translate directly into osmotic potential, because the composition of the dissolved solids must also be known or guessed to get the osmotic potential. But it is a widely used quantity in the water quality business, and to a lesser extent, with aquarium keepers.
The second proxy is conductivity. Pure water has a very low
conductivity, because it has just 2 x 10-7 moles per
liter of charged particles — OH- and H3O+—
that move under an electric field to produce an electrical
current. Most solids dissolved in water break down into charged
particles, such as ions of sodium, calcium, magnesium, chloride,
sulfate, or bicarbonate. The greater the conductivity, the greater
the concentration of charged particles. Conductivity is quite
easily measured to fairly high precision, and so conductivity
meters can be purchased for as little at $15 US.
Although such meters are sold as TDS meters, and return a reading
in units of total dissolved solids, they do not directly measure
total dissolved solids, but return a guess based on likely
composition. This hardly matters: The number of charged
particles is going to be very close to the total number of
particles and thus to the osmotic potential. TDS is simply a
quirky way to express the results. The chief limitation is that
conductivity meters cannot detect non-ionized compounds.
Why does TDS matter? Low TDS will be reflected in low dGH, so if
your dGH is good, your TDS is not too low. High TDS in water with
a reasonable dGH is an indication of brackish or polluted water.
If TDS in your supply water is high, it likely contains excess
sodium, which is hard on plants. Water supplies are noticeably
salty at levels around 200 mg/l of sodium, but drinking water can
be as high as 1000 mg/l before it becomes obviously unhealthy to
drink. Aquarium plants can tolerate some sodium (dwarf hairgrass
actually requires it) but are less tolerant of sodium than humans.
A rise in TDS in otherwise clean aquarium water is regarded as a
sign of high dissolved organics, which are breakdown products of
dead organisms. In a natural environment, dissolved organics are
either scavenged by living organisms or ultimately break down to
carbon dioxide, water, nitrate, sulfate, and other simple ions.
However, some dissolved organics, such as humic acids, are highly
resistant to further decomposition. Dissolved organics are best
controlled with regular water changes.
If total dissolved solids in the supply water are too high, there are three alternatives: Use a different water supply. Run the water supply through a reverse osmosis filter. Run the water supply through a total ion exchanger.
Rainwater. It may be tempting to use collected rainwater as a soft water source. There are some surprising caveats. First, rainwater collected from eaves may contain a fair amount of pollution from asphalt shingles. Metal roofs may add copper or other heavy metals. Tiled roofs are the safest bet, assuming they are not freshly installed. However, rain downwind of industrial areas may contain a surprisingly high amount of sulfuric acid ("acid rain') or heavy metal contamination even if carefully collected. Finally, in some parts of the western U.S., you do not actually own the water rights to rain that falls on your roof. You are technically stealing the water from whoever does (typically a farmer or municipality with water rights further down the watershed), though enforcement against a homeowner collecting a few barrels is likely to be nonexistent. In other words, rainwater is often neither pure nor free.
Reverse osmosis. Reverse osmosis is just that; the use of
high pressure to reverse the process of osmosis, forcing water
from a region of high dissolved solids to a region of low
dissolved solids. This requires adequate pressure and a suitable
membrane. Typical pressure in water supplies is around 65 PSI, so
reverse osmosis based on supply pressure (no supplemental pumping)
can reduce dissolved solids by at most 173 millimoles per liter.
This is normally more than ample, and most home RO systems have no
supplemental pumping. The catch is that flow rates can be quite
slow, so such systems fill a cistern with purified water over the
course of several hours.
The membrane must be permeable to water molecules but not to dissolved solids. Since colloids and some chemical impurities can plug such a membrane, RO systems typically filter the water, then run it through a carbon canister, before running the water past the RO membrane. The water coming out the other side of the membrane will have greatly reduced total dissolved solids. However, RO systems are only about 25% efficient, meaning that for every gallon of RO water produced, three gallons remains with the dissolved solids on the high pressure side of the membrane and must be discarded. This wastes water and is a source of pollution.
Reverse osmosis systems are advertised online for as little as
$135, but over $200 is more typical for a basic system. This
assumes you do the installation yourself.
Ion exchange. The water produced by reverse osmosis still contains some dissolved solids that leak through the membrane. For really pure water, a final stage of ion exchange is sometimes employed. Ion exchange can also be used by itself, though this is an expensive purification method. The water passes through a bed of resin beads, typically sodium polystyrene sulfonate
and polyAPTAC. These have a large surface area over which the water flows. The resin contains sites to which cations (for a cationic resin such as polystyrene sulfonate) or anions (for an anionic resin such as polyAPTAC) can attach.
In a total-exchange system, the cationic resin is initially
loaded with protons and the anionic resin with hydroxide. As the
water flows past, any cations bind to the resin in place of
protons, which enter the water as hydronium. Anions likewise bind
to the resin in place of hydroxide, which also enters the water,
neutralizing the hydronium. This produces water that is almost as
pure as distilled water. The system works best as a mixed bed
system, in which the two kinds of resin beads are mixed together
in a single resin bed. The protons released by the cationic beads
help draw hydroxide from the anionic beads, and vice versa,
increasing the rate and completeness of exchange.
The supply of protons and hydroxyl in the resins is eventually used up. Most mixed bed resins marketed to aquarium keepers and other small-scale users are meant to be discarded when exhausted. They often have some kind of dye indicator so that the resin changes color when it needs to be replaced. Larger commercial mixed-bed systems are designed so that the resin can be recharged. The cation and anion beads are manufactured with different buoyancies, so that back flushing the bed separates the beads, and the cation beads can then be recharged with a strong acid (typically hydrochloric acid) and the anion beads with a strong base (typically sodium hydroxide). Such rechargeable mixed bed systems are not practical for most home uses.
One could also maintain separate anion and cation beds, running
the water through them in sequence. This would simplifying
recharging. Such a system would be inherently less effective than
a mixed bed system, but might work well if several cation and
anion resin beds were alternated. This would be an interesting DIY
project.
For the serious home aquarium keeper who needs a modest supply of very soft water, a home reverse osmosis system is probably the cheapest and simplest option.
The output of a RO or ion exchange system will be devoid of
dissolved solids. It must have electrolytes added to make it
suitable for fish and plants. The easiest course of action is to
use the RO water to dilute tap water to the desired hardness. If
this is not feasible, then several commercial products are
available for this purpose and any is likely to be satisfactory.
Those intended for reefs will include trace elements not needed in
a planted aquarium, so it is cheaper to use one intended for
freshwater aquariums. The less expensive contain sodium chloride,
which is fine for fish but may add too much sodium for plants. The
more expensive formulations contain only potassium, which is great
for plants but must be tracked and possibly supplemented in a
heavily planted aquarium. Fish need sodium but can obtain what
they need from their food, particularly if you give them an
occasional treat of brine shrimp.
If you want to add your own electrolytes to RO water, the steps are:
A plausible recipe would be to add 72 mg/l of finely powdered calcium carbonate to the water, to bring the carbonate hardness up to 4 dKH. Next is 73 mg/l of Epsom salt (hydrated magnesium sulfate) to achieve the 4:1 ratio of calcium to magnesium. This produces water with a dGH of about 5, which may be just fine. If not, calcium chloride and magnesium sulfate may be added in about a 3:1 ratio by weight to raise the general hardness further. Then add 19 mg/l of potassium chloride. Add no sodium chloride, but treat your fish to an occasional snack of brine shrimp.
Experimental aquarium
chemistry app
Next chapter: Photosynthesis
Copyright ©2019 Kent G. Budge. All rights reserved.